-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(2): 6-11
doi: 10.5923/j.chemistry.20120202.02
Synthesis and Characterization of γ-irradiated PVA/PEG/CaCl2 Hydrogel for Wound Dressing
Joydeep Dutta
Department of Chemistry, Disha Institute of Management and Technology, Satya Vihar, Raipur, 492101, India
Correspondence to: Joydeep Dutta , Department of Chemistry, Disha Institute of Management and Technology, Satya Vihar, Raipur, 492101, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The mixtures of polyvinyl alcohol (PVA) and polyethylene glycol (PEG) with calcium chloride (CaCl2) were exposed to gamma-irradiation to synthesize transparent hydrogels for wound dressing. In this system, CaCl2 was used not only as a gelling material but also as a plasticizer. The presence of CaCl2 enhances the effects of PEG, this synergistic effects give births a new hydrogel with improved characteristics. The water absorption capacity of the hydrogel was in the range of 350-375% after immersion in normal saline for 72 h. In vitro weight loss experiments were conducted at 37 oC in order to simulate the use of dressing on wounded skin. The enhanced thermal stability of the hydrogel was confirmed by thermogravimetric analysis (TGA). FTIR studies confirmed the cross-linking between PVA and PEG in the hydrogel. Furthermore, the microbe penetration test of the hydrogel was carried out to confirm its impermeability to bacteria. The effect of hydrogel on cell proliferation was also evaluated to ensure that the hydrogel does not release materials which might be deleterious to cell proliferation. The results showed that the hydrogel could be considered as a potential wound-dressing material.
Keywords: Hydrogel, Radiation, Polyvinyl Alcohol, Polyethylene Glycol, Calcium Chloride, Wound Dressing
Article Outline
1. Introduction
- In the recent past, hydrogels have received increasing attention due to their potential application in wound healing [1-4]. According to the old concept of wound healing, the use of cotton and gauze was quite significant to keep the wound dry. It has been observed by Winter[5] that a scab formation occurs when a wound is left open without any dressing thereby decreasing the rate of wound epithelization. He showed that a moist wound dressing can remarkably increase the rate of epithelization by preventing the scab formation. The hydrogels are suitable for absorbing exudates and serve as temporary physiological covers. It also provides efficient shielding as well as protects the wound from mechanical trauma. PVA is an important material because it is non-toxic, non-carcinogenic, biodegradable, biocompatible, water soluble, and non-expensive polymer[6,7]. It has been extensively commercialized and studied in the chemical and medical industries[8] for the productions of fibers, films, coatings, cosmetics, pharmaceuticals, etc. PVA-based hydrogels are promising wound dressing materials because of their permeability to small molecules, impermeability to bacteria, soft-consistency, low interfacial tension, high water content and transparency[9]. Hydrogels can be prepared in various ways. Motoyama et al.[10] demonstrated the preparation of PVA hydrogel by using covalent cross-linking agent such as glutaraldehyde into aqueous solution of PVA. In another study, Singh et al. [11] reported a PVA hydrogel chemically cross-linked with an aldehyde such as formaldehyde, glutaraldehyde, terephthalaldehyde, and hexamethylenediamine to increase the gel strength. The disadvantages of such hydrogels include presence of residual cross-linking agents in the hydrogel which could be toxic to the tissues. Further, the elimination of the residuals increases the production cost. Varshney[12] demonstrated the synthesis of PVA-based hydrogel by gamma-irradiation technique. Besides, the above hydrogel also contains agar and carrageenan as other components, and is used for treating burns, non-healing ulcers of diabetes, and other external wounds etc. The use of agar as a mechanical strength enhancing agent in hydrogel synthesis makes it susceptible to microbial contamination. In another study, Mirzan et al.[13] reported the synthesis of gamma-irradiated polyvinyl alcohol-polyvinyl pyrrolidone (PVA-PVP) blended hydrogel. They investigated the gel fraction, mechanical properties, water content and water absorption capacity of the hydrogel for wound dressing. Many of the researchers have reported the synthesis of PVA-based hydrogels by using freeze-thaw method[14-16]. Altering process parameters, such hydrogels can be made transparent by using this method. In a study, Kim et al.[17] reported the development of polyvinyl alcohol-alginate gel-matrix-based wound dressing containing nitrofurazone by using freeze-thaw method. In another study, Nho et al.[18] reported a novel PVA/PVP/glycerin/antibacterial agent hydrogel for wound dressing by using gamma-irradiation followed by freeze-thaw method. Freeze-thaw is a very expensive and time-consuming method whereas gamma- irradiation method accomplishes gel formation and sterilization simultaneously in one step.Bearing in mind that gamma-irradiation reduces the production cost as well as to avoid chemical cross-linkers, we have developed a novel hydrogel which is composed of polyvinyl alcohol, polyethylene glycol, and calcium chloride. To the best of our knowledge, the development of novel PVA/PEG/CaCl2 hydrogel has not been reported so far. This hydrogel as a wound dressing material has been demonstrated by its good water absorption capacity, high water content and minimum weight loss. Its impermeability to microbial contamination and its non-cytotoxic nature reflect its advantages as a suitable wound dressing material.
2. Experimental
2.1. Preparation of Gel Systems
- First of all, CaCl2 (Sigma, Germany) was dissolved in de-ionized water system followed by the addition of approximate amounts of PEG 4000 (Thomas Baker, Mumbai, India) to it. Once, the PEG 4000 got into the solution, 20% w/w of PVA M. W. 14,000 (West Coast Laboratories, Mumbai, India) was directly added to the system. Then the entire mixture was subjected to autoclave for 1h for complete dissolution. After autoclaving, the solution was deaerated and poured into trays. The trays were then inserted into plastic pouches and sealed. Finally, the trays containing solution were exposed to gamma-irradiation at 25 kGy for the formation of hydrogels.
2.2. Characterization
- For swelling experiments, the hydrogel samples were immersed in excess normal saline solution at 37℃. At predetermined time point, the hydrogels were taken out and weighed after removal of surface water with cotton cloth. Swelling was calculated as follows
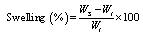 | (1) |
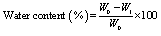 | (2) |
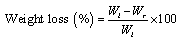 | (3) |
3. Results and Discussion
- Introduction of PEG into the macromolecular chain of PVA in presence of CaCl2 improved some of the characteristics of the final hydrogel (Scheme 1). The mechanism for the formation of PVA hydrogel using gamma-irradiation is already known[21]. Irradiation of aqueous PVA solution results in a chemical cross-linking of the polymer chains by forming covalent bonds.
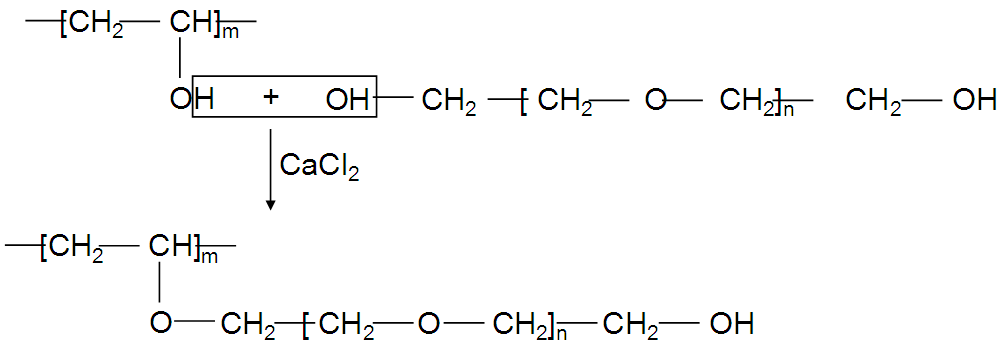 | Scheme 1. Interaction between PVA and PEG in presence of CaCl2 |
3.1. Swelling Study of Hydrogels
- The gel thickness was optimized by performing swelling measurements only for PVA hydrogels of different thicknesses namely 3 mm, 4 mm and 5 mm (Fig. 1). As can be seen from the Fig. 1, there is no marked difference of the swelling capabilities of the hydrogels of varying thicknesses upto 24 h. After that the degree of swelling of the hydrogel of 3 mm thickness remarkably increases over time and reaches equilibrium at 72 h. On equilibration, the swelling increased to more or less 300%. On the basis of the degree of swelling, 3 mm thickness of the hydrogel was optimized for wound dressing.
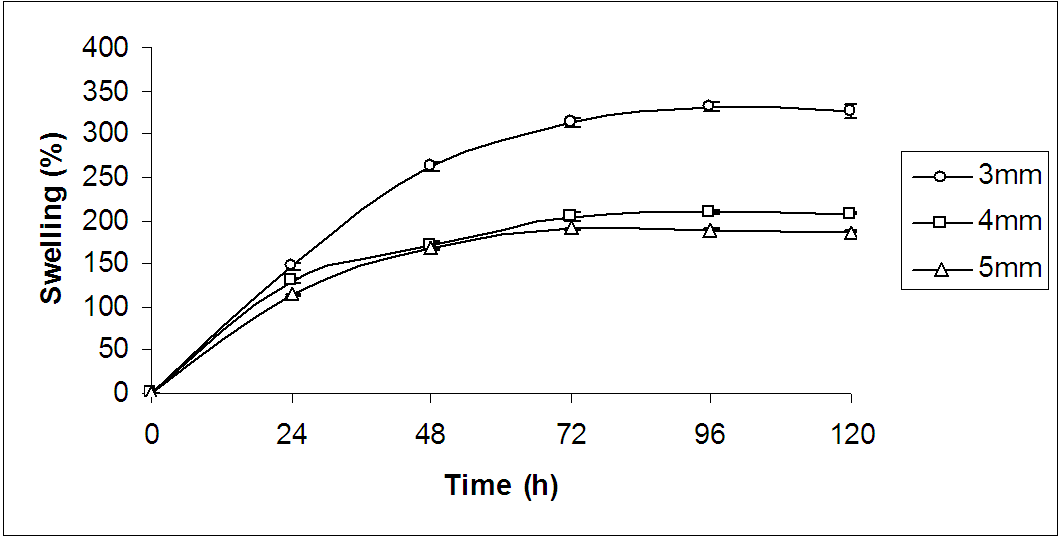 | Figure 1. Time course of swelling change in normal saline solution at 37℃ for only PVA hydrogels of various thicknesses |
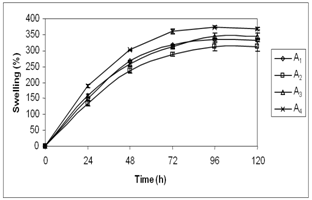 | Figure 2. Swelling behaviour of hydrogels of different compositions |
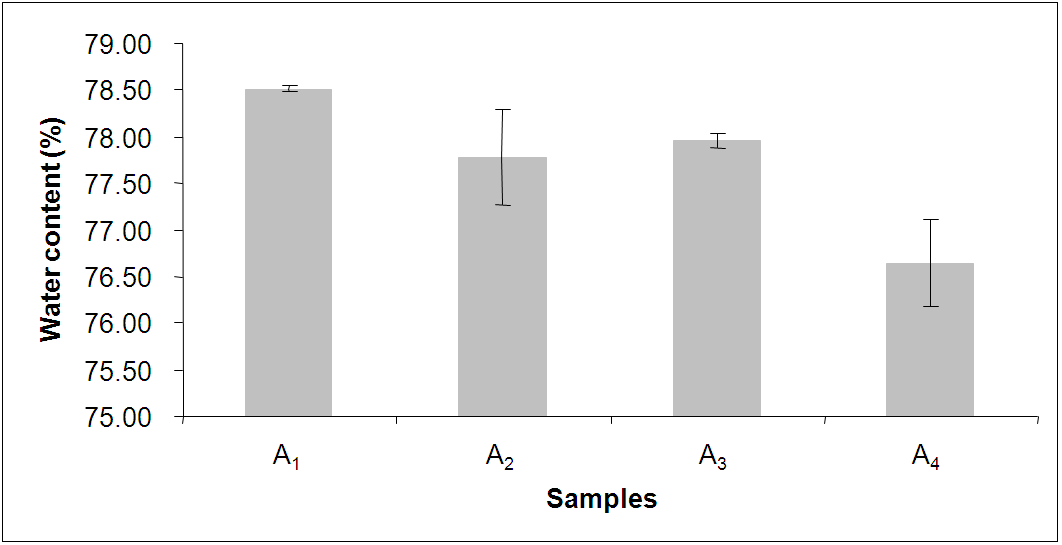 | Figure 3. Experimental data of water content of hydrogels of different compositions |
3.2. Water Content Analysis
- As shown in Fig. 3, it was observed that the water content of the hydrogels of different compositions to be in the range between 76% and 79%. Mirzan et al.[13] showed that higher the water content in the PVA-PVP hydrogel, lesser is the ability to absorb more water. Here, the A4 hydrogel possesses the least water content i.e. 76.65 ± 0.66 % but the highest water absorption i.e. 359.78 ± 13.33% after 72 h immersion in normal saline compared to other hydrogels which agrees the above statement.
3.3. Weight Loss Study
- Weight loss experiments were conducted at 37℃ in order to simulate the use of dressing on wounded skin. Fig. 4 shows the relative weight loss of the hydrogels of different compositions. However, the weight loss of the A4 hydrogel is 17.64 ± 0.79% at 72 h whereas in other hydrogels the weight loss is in the range of 22-25%. This phenomenon can be explained as follows: 1) due to hydrophilic nature, PEG attracts more water molecules towards itself as compared to PVA, and 2) due to hygroscopic nature of CaCl2, and it holds maximum numbers of water molecules and does not allow them to escape from the surface of the A4 hydrogel. Surprisingly, the only presence of PEG or CaCl2 in the hydrogel does not exhibit the said phenomenon. Therefore, it is assumed that this interesting characteristic of the A4 hydrogel may accelerate the healing process by providing a moist environment that leads to rapid granulation and re- epithelization; and concurrently, may prevent the wound from scab formation. Here, it is quite noteworthy to mention that the moist environment is conducive for faster wound- healing.
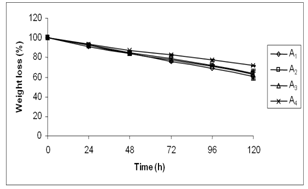 | Figure 4. Weight loss of hydrogels of different compositions |
|
3.4. Tensile Strength and Elongation
- As a consequence of their high water content, hydrogels lose mechanical strength which makes it difficult to handle. Tensile strength and elongation at break of the A4 hydrogel were measured to find its suitability as a wound dressing material. The results are shown in Table 1. It shows that the tensile strength and elongation at break of the A4 hydrogel are 16 × 10-2 Kgf/cm2 and approximate 233% respectively. Thus, the A4 hydrogel possesses sufficient mechanical strength for its easy handling during its application on the wound.
3.5. Thermogravimetric Analysis (TGA)
- Thermal decomposition behaviour of the A4 hydrogel was determined by thermogravimetric analysis. Fig. 5 shows that initial decomposition starts for (a) PVA at 243℃ and (b) for PEG at 328℃. In Fig. 5 (c), it was found that weight suddenly dropped to 76% was due to loss of water content in the A4 hydrogel. The initial decomposition temperatures of both PVA and PEG were higher than those which were shown in their individual therograms, indicating that PEG in presence of CaCl2 improved the thermal stability of the hydrogel, because the hydroxyl groups of PEG formed hydrogen bond cross-linking with hydroxyl groups of PVA. This restricted the chain movement during thermal treatment which is confirmed by FTIR spectroscopy as discussed below.
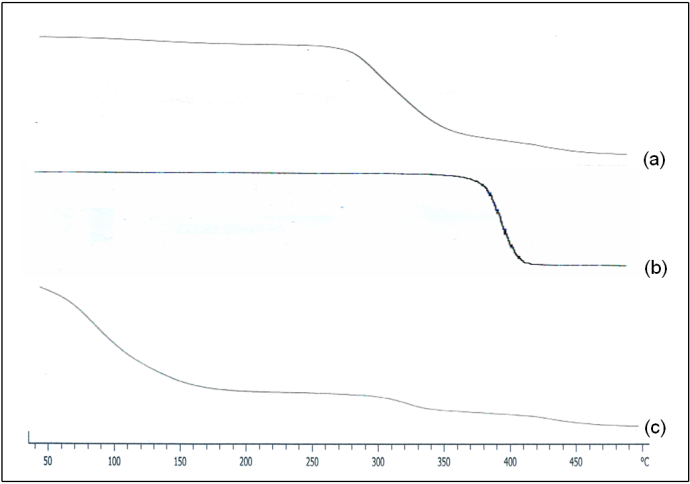 | Figure 5. Thermograms of (a) PVA, (b) PEG, and PVA/PEG/CaCl2 hydrogel |
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
- In Fig. 6 (c), the FTIR spectrum of the A4 hydrogel exhibits several characteristic bands of stretching and bending vibrations of O-H, C-H, C=C and C-O groups. Two broad and strong bands were observed at 3318 and 657 cm-1 corresponding to O-H stretching frequency. Typically, strong hydroxyl bands for free alcohol (nonbonded –OH stretching) and hydrogen bonded bands were found in the respective region between 3600-3650 cm-1 and 3200-3500 cm-1[23-25]. The respective characteristic absorption bands at 3408 and 3391 cm-1 for hydroxyl groups were found in the FTIR spectra of PVA and PEG shown in the Fig. 6 (a) and 6 (b). Therefore, shifting of the band to a lower wave number i.e. at 3318 cm-1 as well as band widening clearly indicates the presence of hydrogen bond in the A4 hydrogel. Again, in Fig. 6 (c), the band observed at 2952 cm-1 indicates an asymmetry in stretching mode of CH2 group. A weak band was observed at 2137 cm-1 and had been assigned to the combination frequency of (CH + CC). The band at 1639 cm-1 has been assigned to C=C stretching mode. Two bands observed at 1417 and 1380 cm-1 have been attributed to bending modes of CH2 groups. A weak band at 1326 cm-1 was assigned to the combination frequency of (CH + OH) group. The FTIR spectrum for the system PVA/PEG/CaCl2 showed a characteristic absorption band for –C-O-C- stretching vibration at 1094 cm-1. This again confirms the cross-linking between PVA and PEG in this hydrogel.
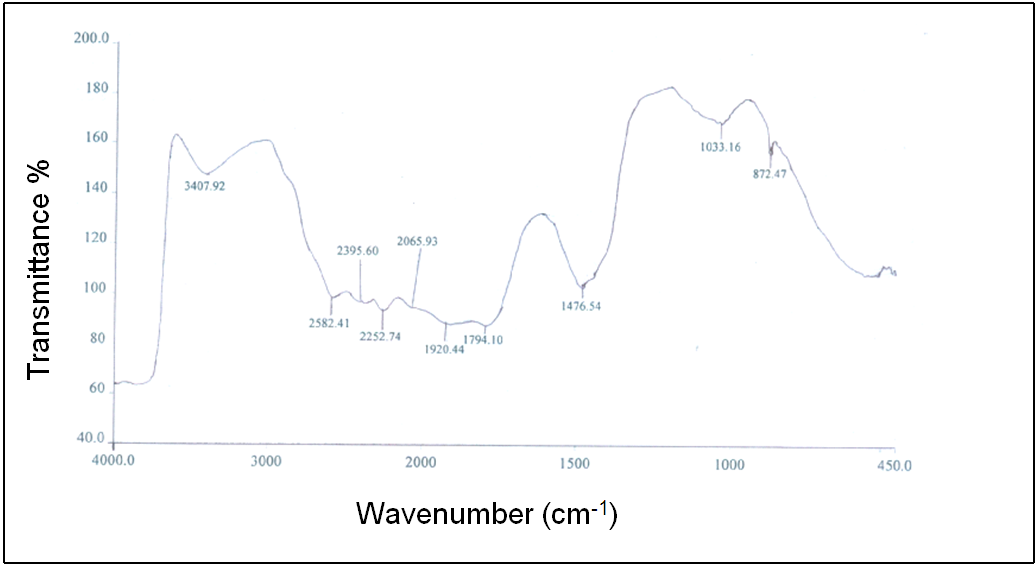 | Figure 6 (a). FTIR spectrum of PVA |
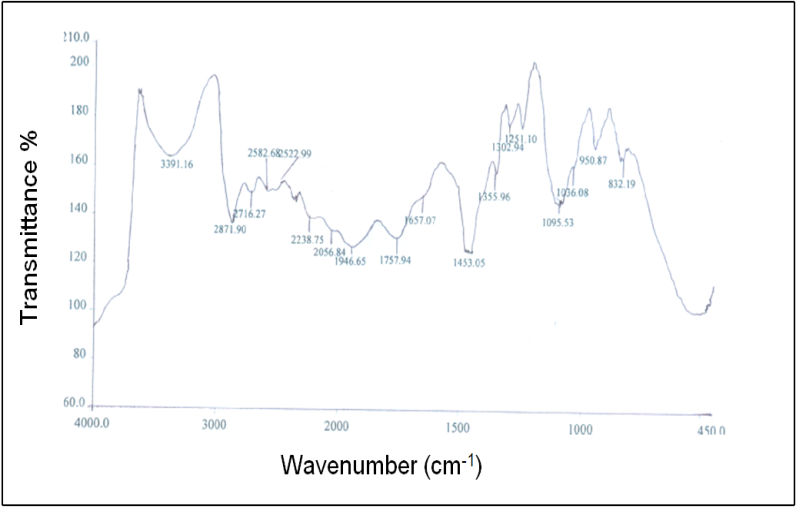 | Figure 6 (b). FTIR spectrum of PEG |
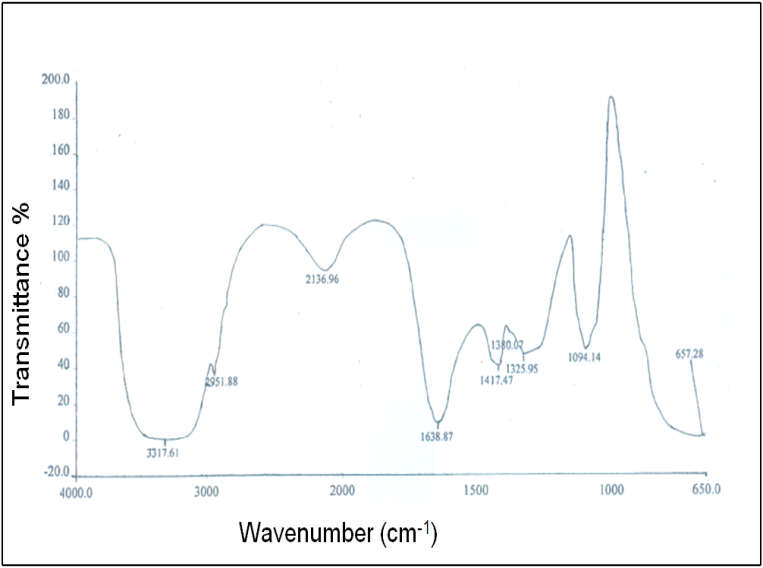 | Figure 6 (c). FTIR spectrum of PVA/PEG/CaCl2 |
3.7. Microbe Penetration Test
- Based on the microbe penetration test it was found that no bacteria passed through the A4 hydrogel into sterile media even after 48 h. This indicated that the 3 mm thickness of the hydrogel dressing could protect the wound from getting infected. In another study, Kokabi et al.[26] also reported that 3 mm thickness of PVA-clay nanocomposite hydrogel protected wound from the same, while Purna et al.[27] had shown that the use of 64 layers of gauze was inefficient to prevent microbial penetration. This ability of the hydrogel would protect the wound from infection.
3.8. Cytotoxicity Studies
- Cytotoxicity of A4 hydrogel was indirectly assessed by studying the effect of hydrogel exposed media on keratinocytes and fibroblasts proliferation using the MTT method which is shown in Fig. 7. No significant difference in the proliferation of cells treated with either control media or hydrogels exposed media was observed. Similar rate of proliferation in keratinocytes and fibroblasts indicated that the hydrogel was non-toxic and its application on the wound bed would not interfere with cell proliferation. In another study, Sirousazar et al.[28] also reported that polyvinyl alcohol/clay nanocomposite hydrogel is non-toxic and biocompatible wound dressing. Therefore, it can be said that in vitro cytotoxicity studies give the preliminary authentication of the material to be used as wound dressing is non-toxic and biocompatible as well.
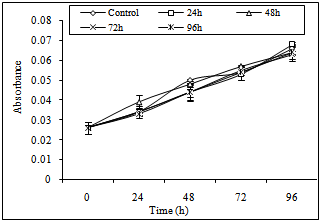 | Figure 7 (a). The effect of hydrogel exposed media on keratinocytes proliferation |
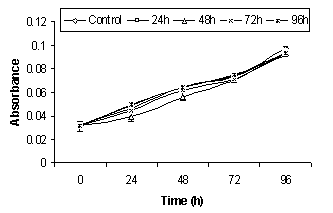 | Figure 7 (b). The effect of hydrogel exposed media on fibroblasts proliferation |
4. Conclusions
- The above studies demonstrated that the PVA/PEG/CaCl2 (A4) hydrogel of 3 mm thickness was suitable for providing a moist environment over the wound bed because the percent weight loss of the A4 hydrogel was found to be less than 19% at 72 h whereas in other hydrogels the weight loss was between 22% and 25%. In addition, suitable fluid uptake efficiency (350-375% after immersion in normal saline for 72 h) would enable the said hydrogel to provide an adequate moist environment that leads to rapid granulation and re- epithelization to wound closure. The enhanced thermal stability of the hydrogel indicated that both PVA and PEG were cross-linked together through hydrogen bond. This was again confirmed by FTIR study. Microbial penetration test revealed that the A4 hydrogel could be considered as a good barrier against microbes. The cytotoxicity studies showed no inhibition on cell proliferation. Furthermore, the transparent appearance of the hydrogel would obviate the need for frequent dressing changes to monitor the wound from outside. Finally, the above properties of the A4 hydrogel suggest that it can be used as a wound dressing in practical wound management.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML