Ohochuku N. Stephen
Department of Chemistry, Rivers State University of Education, PMB 5047, Port Harcourt, Nigeria
Correspondence to: Ohochuku N. Stephen, Department of Chemistry, Rivers State University of Education, PMB 5047, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
A chemical principle supporting the priority sequence rule in the systematic nomenclature of organic compounds was sought for. The positions of functional groups containing carbon, hydrogen and oxygen in the priority sequence were examined for possible chemical criteria that could be used to buttress the positions of the functions. Oxidative sequence emerged as possible chemical criteria. This chemical principle was applied to other functions containing nitrogen as well and the result was highly positive. However the position of -C≡C- was not favored, this is commented upon. In general oxidative sequence supports the priority sequence and provides a fast means of deciding which function has higher priority instead of resorting to rote learning.
Keywords:
Oxidative Sequence, Chemical Criteria, Priority Sequence
Cite this paper: Ohochuku N. Stephen, Oxidative Sequence: A Chemical Principle in Support of priority Sequence Rule in Systematic Nomenclature of Organic Functional Groups, American Journal of Chemistry, Vol. 2 No. 1, 2012, pp. 11-12. doi: 10.5923/j.chemistry.20120201.03.
1. Introduction
The nomenclature of chemical compounds, especially organic, before the advent of the International Union of Pure and Applied Chemists (IUPAC) system of nomenclature witnessed various patterns commonly classified as common or trivial apart from other ways such as trade names. Inherent in these patterns or systems is the possession of more than one name for some compounds. As more organic compounds were discovered and or synthesized, so the confusion created by trivial names skyrocketed. To curb the menace being created by the trivial names, chemists looked for a method of nomenclature that will assign only one name to one organic compound. This search resulted to the formation of rules now known as IUPAC system of nomenclature. The major aim of IUPAC system of nomenclature is to give an organic structure only one name by which the structure can be written and vice versa by any chemist anywhere in the world; but the view has been expressed [1] that while it is true that no two organic compounds will have the same name, it is hard to believe that an organic compound could not possess more than one IUPAC name. The latter is true considering the complexity and diversity of organic compounds and the IUPAC rules permitting the use of substitutive and functional class nomenclatures for compounds of some families. A brief summary of the rules of the IUPAC system of nomenclature are (i) the choice of the names of the saturated acyclic hydrocarbons to provide the stem in the naming system; (ii) the designation of some functional groups as principal functions that form principal or main families, each family being given a suffix that identifies the family; (iii) generation of priority sequence amongst these main functional groups: this is very important when any two or more of these different principal functions occur in a single molecule; (iv) classification of the remaining functional groups as substituents and (v) providing suitable prefix for each type of functional group (main or not) where applicable, when the function occurs as substituents.
2. Methodology
Consider the group of functions containing carbon and or oxygen which are the alkene (>C=C<), alkyne (-C≡C-), alcohol (-OH), carbonyl (>C=O) and the carboxylic acid (-CO2H) (the esters, and acid anhydrides are omitted in this discourse). Apart from the alkyne, the rest in this listing conform to the priority sequence of the IUPAC rule. This sequence just above is in increasing order of oxidation. Hence the increasing priority sequence can be said to result from the increasing oxidative sequence as illustrated below.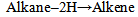 | (1) |
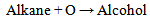 | (2) |
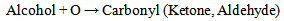 | (2a) |
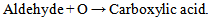 | (2b) |
Equations 1 to 2b, starting with an alkane, its oxidation by loss of two Hydrogen atoms (2H) gives an alkene. While the absorption of an atom of oxygen (O) by an alkane gives an alcohol, the loss of 2H by an alcohol gives a carbonyl and the gain of one O by a carbonyl (aldehyde) gives a.carboxylic acid. These are illustrated below with ethane.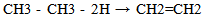 | (3) |
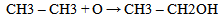 | (4) |
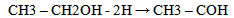 | (4a) |
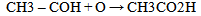 | (4b) |
Oxidative sequence is also applied to functions containing nitrogen (CN, NH2, etc) as well in the following manner.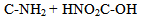 | (5) |
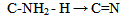 | (6) |
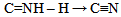 | (7) |
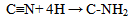 | (8) |
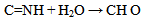 | (9) |
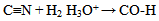 | (10) |
From equations 5 to 10 (8 and 10 being the reverse of oxidation) the increasing priority sequence within OH, CN, NH2, CHO can be deduced. Also the oxidative sequences 9 to 14 enable the prioritisation of C=C, C≡C, OH, -CHO and –CO-C to be done.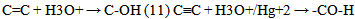 | (12) |
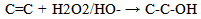 | (13) |
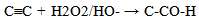 | (14) |
3. Results and Discussions
Oxidative sequences 3 and 4 confirm the positions of C=C, OH, -CO-H and COOH in the IUPAC sequence rule while 6 and 7 show that CN > C=NH > NH2, hence CN > NH2. Sequence 5 shows that OH > NH2, therefore CN > -CO-H > OH > NH2 utilizing 10 (a reduction). These priorities tally with the IUPAC list.Oxidative sequences (11) and (12) show that C=C is converted to alcohol under the same condition where C≡C gives carbonyl which show C≡C > C=C and this is further supported by the oxidation sequence C=C –2H → C≡C. This sequence C≡C > C=C though perfectly in order is contrary to that in IUPAC which all chemists have accepted. It has gained wide publicity and is in use in many texts2, 3. It also has been used to compile chemical abstract and other data bases so the IUPAC sequence C=C > C≡C remains.The part played by increasing oxidative sequence in the prioritization of functional groups is indicated by the statement [4] that in a compound possessing more than one functional group, at least one of which is oxygen, the most oxidised functional group determines the name ending and the other functions are treated as substituents. This is exemplified by H2N-CH2-CH2-OH (2-Aminoethanol), HC(OH)= C(NH2)-CO-H (2-Amino-3-hydroxy-2-propenal), CH3-CO- CH2-CO2H (3-ketobutanoic acid). The extension of the use of oxidative sequence to other functions not containing oxygen shows its broad application and a rule emanating from the oxidative sequence is that a functional group that gives another functional group by oxidation has lower priority to the function it produces, hence the increase in priority from alcohol to carbonyl to carboxylic acid. It follows that two functions (similar or not) of different priorities oxidise to different functions which bear the same priority sequence as the substrates. Typical example is p-ol (greater than s-ol) on oxidation gives aldehyde which has higher priority than ketone given by s-ol.
4. Conclusions
The above shows that the priority sequence of the functional groups for purposes of systematic naming of organic compounds is supported by oxidative sequence and therefore is based on chemical facts. Oxidative sequence provides a fast means of deciding which function has higher priority instead of resorting to rote learning.
References
| [1] | F. A. Carey. Organic chemistry. 6th edition. Mc Graw Hill, NY, 2000. p77 |
| [2] | A. Bahl and B. S. Bahl. Advanced Organic chemistry 2006 reprint. S. Chand and Company Ltd, 7361; Ram Nagar, New Delhi 110055. India, 2006 p175 |
| [3] | D. C. Neckers and M. P. Doyle, Organic Chemistry, John Wiley & Sons, Inc. NY, 1977. p163 |
| [4] | B. M. Daniel and R Viateur. Foundations of College Chemistry, 3rd ed. John Wiley & Sons N.Y. 1980. Pp 547-8 |

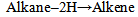
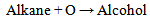
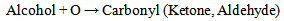
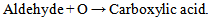
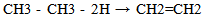
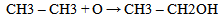
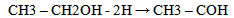
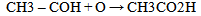
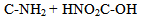
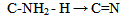
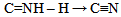
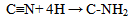
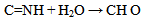
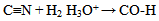
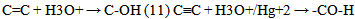
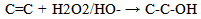
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML