-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(1): 7-10
doi:10.5923/j.chemistry.20120201.02
Soap Production from Agricultural Residues - a Comparative Study
L. E Yahaya, A. A Ajao, C. O Jayeola, R. O Igbinadolor, F. C Mokwunye
End-Use Research Division, Cocoa Research Institute of Nigeria, Ibadan, Nigeria
Correspondence to: L. E Yahaya, End-Use Research Division, Cocoa Research Institute of Nigeria, Ibadan, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
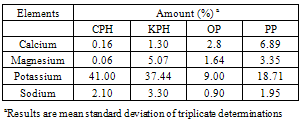
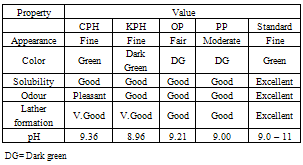
Four agricultural by-products namely Cocoa pod husk (CPH), Kola pod husk (KPH), Plantain peel (PP), and Orange peel (OP) were examined for their potential as alternative source of potash for soap production. These materials were ashed and the samples were subjected to hot aqueous extraction.Extracts from the ashed samples were characterized of their inorganic elements viz calcium, sodium, potassium and magnesium. Results indicated that these samples contains appreciable amount of potassium, a precursor material for soap production with CPH giving the highest value (41%).The percentage amount of the potash is of the order CPH > KPH > PPA > OPA.Liquid soaps were thus prepared from the extracts of CPA, KPH, PPA and OPA. Physical and chemical characteristics of the resulting soap products were carried out. The overall quality of the products compared well with that of the standard with exception of OP which showed little variations.Indications therefore abounds that these agricultural residues are suitable material or alternatives for convectional alkalis for soap production.
Keywords: Agricultural Residues, Soaps, Alkalis, Chemical Characteristics
Cite this paper: L. E Yahaya, A. A Ajao, C. O Jayeola, R. O Igbinadolor, F. C Mokwunye, Soap Production from Agricultural Residues - a Comparative Study, American Journal of Chemistry, Vol. 2 No. 1, 2012, pp. 7-10. doi: 10.5923/j.chemistry.20120201.02.
Article Outline
1. Introduction
- There has been high demand for surface active agents like soap over the years. This could be probably as a result of its usage virtually in all household affairs. According to McCutheon (1974), soap which is alkali salts of long chain carboxylic acids belongs to the class of anionic surfactant.Surfactants have been defined as an organic compound which alters the conditions prevailing at the boundaries between different phases in a system e.g between water and oil in the formation of emulsion or between water and air in the case of foams. (McCutheon, 1974).These compounds (surfactants) consist of two parts: a hydrophilic portion which include a long hydrocarbon chain and a hydrophobic portion which renders the compound sufficiently soluble or dispersible in water or other polar solvent. The combined hydrophilic and hydrophobic moieties of the compound makes it active and thus able to concentrate at the interface between surfactant solution and another phase such as air, soil and textile or other substrate to be cleaned. (Shreves, 1967). Soap forms the largest group of the detergents that are in common use today as they constitute about 95% of all the detergents(Wilkinson,1974). A soap molecule consists of long hydrocarbon chain with a carboxylic acid group on one end which is ionic bonded to a metal ion, usually a sodium or potassium. The hydrocarbon end is non polar and is soluble in non polar substances such as fats and oils, and the ionic end (the salt of a carboxylic acid) is soluble in water (structure represented in scheme 1).Saponification which is the basic reaction leading to the formation of soaps employs the use of fatty acids and alkalis such as potassium and sodium hydroxide. The pressure on the use of these chemicals for soap production has resulted in its exorbitant price. Efforts have therefore been geared towards sourcing for alternatives. Majority of agricultural materials are embedded with these alkalis which can be used if properly harnessed. Some of these materials include cocoa pod husk (CPH), kola pod husk (KPH), orange peel (OP), and plantain peel (PP).The availability of these materials has also rekindled the interest of this study; for example, it has been estimated that that Nigeria produces about 185,000 metric tones of cocoa (Theobroma cacao) per annum (CAN, 2006). The implication of this is that heaps of the pod husks abound as wastes in the plantation which constitute menace to plantation crops. Plantain (Plantago sp.) is also a common staple food in Africa and its production figure is enormous. The peels are often disposed as wastes in streets. Orange, (Citrus sinensis) is a major citrus cultivated in Nigeria especially in the south western part of the country. All these by-products are often regarded as wastes.In our effort to add value to these plantation crops, the seemingly waste products from these crops were tapped by extracting potash and using the potash for soap production.This paper thus report a comparative study of soap produced from the by-products of these agricultural residues. CH3-CH2-CH2- CH2(CH2)9CH2- C O O− Na+ionic end(Soluble in nonpolar substances) (Soluble in water)Scheme 1. Schematic representation of a typical soap.
2. Materials and Methods
2.1. Materials
- Materials used for this experiment included diethyl ether, hydrochloric acid, acetone, ethanol and potassium hydroxide. Also used were palm kernel oil, flame photometer, kiln furnace, pH meter ( kent EK 7020). etc.
2.2. Preparation of Potash
- Dried cocoa pod husk (CPH) and kola pod husk (KPH) were obtained from the Cocoa Research Institute of Nigeria, Ibadan, while plantain peel (PP) and orange peel (OP) were obtained from the open market. CPH, KPH, OP and PP were subjected to the process of ashing. In this case, large quantity of each sample was introduced into an incinerator and heated at an elevated temperature of about 450oC for one hour. Known weights of the ashed samples were boiled for an hour. This was followed by filtration. The filtrate which contains the mineral elements were taken and assayed for calcium, magnesium, potassium and sodium using flame photometer according to AOAC methods (AOAC, 1990).
2.3. Liquid Soap Preparation
- Preparations of liquid soaps from these agricultural wastes were carried out according to standard methods (Kirk-Othmer, 1967; Chaco et al, 1993; and Yahaya et al., 2002). Each extract was concentrated to correspond to 38 Baume, a value equivalent to alkali concentration for convectional soaps. For each sample, one part of palm kernel oil was reacted with two parts of the extract with constant agitation until a semi-solid mass was obtained. This was allowed to stand for 24 hours after which they were dissolved liquid of desired viscosity.
2.4. Evaluation of the Detergency of the Soaps
- The performance characteristics of the resulting liquid soaps were determined by carrying out the lather test according to prescribed method (Chaco, et al 1993). In a typical experiment, 0.02kg each of the soap samples was dissolved in 0.1dm3 in boiling water. A similar solution of synthetic detergent supplied was also prepared. Drops of oils were introduced into the test tubes.0.1dm3 of distill water to one, 0.1dm3 of prepared soap solutions to others and 0.1dm3 of detergent solution to the third test tubes. These tubes were agitated vigorously while in closed state. The formations of lather were examined and compared. The emulsifying ability, i.e., how well the oil was converted to the colloidal state was noted. The pH of the resulting liquid soaps was measured using a pH meter.
2.5. Chemical Characteristics of the Liquid Soaps
- The chemical characteristics, hence the quality of the resulting soaps were evaluated as follows; free fatty acid was determined titrimetrically, unsaponified neutral fat by saponification and then extraction, free alkali and neutral fat by standard methods (AOCS, 1965; AOAC, 1990; ASTM, 1971).
3. Results and Discussions
3.1. Mineral Elements from Agricultural Residues
|
3.2. Physical and Chemical Characteristics of the Soaps
|
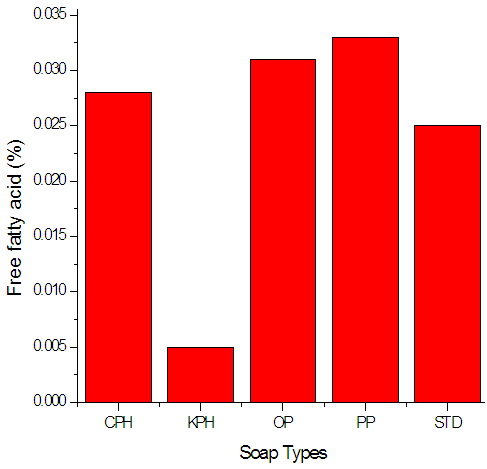 | Figure 1. Free fatty acid of the soap variety |
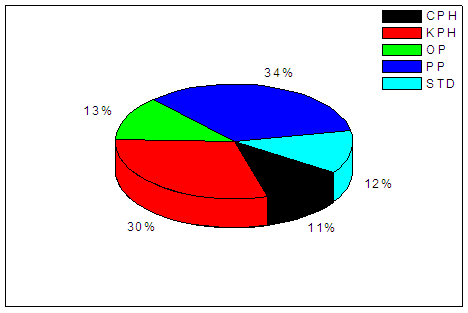 | Figure 2. Free alkali (% K2O) of the different soap types |
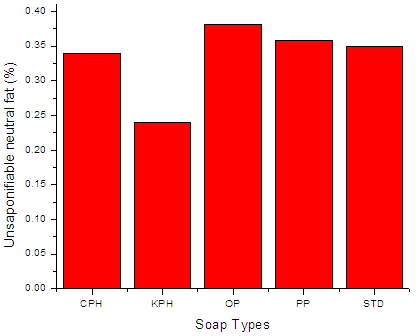 | Figure 3. Unsaponifiable neutral fat of the soap variety. |
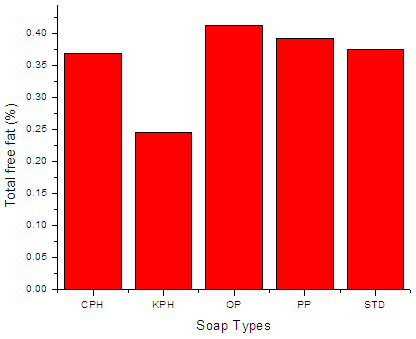 | Figure 4. otal free fat of the soap variety. |
4. Conclusions
- From this study, it was clear that most of these agricultural material contains appreciable amount of potash which can be harnessed for surfactant production.
ACKNOWLEDGEMENTS
- The authors wish to thank the laboratory technologists that contributed to this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML