-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2012; 2(1): 1-6
doi:10.5923/j.chemistry.20120201.01
3-(4-Bromobenzoyl)prop-2-enoic acid as Precursor in Synthesis of Some Important Heterocyclic Compounds
Maher A. El-Hashash, Sameh A. Rizk, Ebtessam A. Ahmed
Chemistry Department, Faculty of Science, Ain Shams University, Abbassia, Cairo, Egypt
Correspondence to: Sameh A. Rizk, Chemistry Department, Faculty of Science, Ain Shams University, Abbassia, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present work deals with the generation and synthesis of different heterocycles such as 2-pyrimidine thione, pyrane, pyridine and phthalazinone derivative via the treatment of 3-(4-bromobenzoyl)prop-2-enoic acid with thiourea, ethylcyanoacetate malononitrile& acetylacetone in presence of amm.acetate and/or piperidine respectively.Additionally, utility of 2-amino-3-cyano-4-carboxy-6-(4-bromophenyl)3,4-dihydropyridine (4)as a key starting material to synthesize some important heterocycles include fused pyrido-pyrimidine,fused pyrido- pyridazinopyrimidine and interesting diheterocyclic amines.
Keywords: 3-Aroyl prop-2-enoic acid, pyrrole, furan, Pyridone, Ethylnicotinate, pyrane, phthalazinone, 2-pyrimidine thione, pyridopyrimidine, pyridazino-pyrimidine
Cite this paper: Maher A. El-Hashash, Sameh A. Rizk, Ebtessam A. Ahmed, 3-(4-Bromobenzoyl)prop-2-enoic acid as Precursor in Synthesis of Some Important Heterocyclic Compounds, American Journal of Chemistry, Vol. 2 No. 1, 2012, pp. 1-6. doi: 10.5923/j.chemistry.20120201.01.
1. Introduction
- It has been reported [1,2] that -aroylacrylic acids were used as inhibitors of phospholipase from snake venom and from procaine pancreas, also they have antibacterial activities [3,4], which prevented the growth of staphylococcus aureus, beside their anti-proliferative [5] action against human cervix carcinoma. Recently [6,7] 3-(4-acetamido)and\or 4-chloro benzoyl prop-2-enoic acid were used in the synthesis of some heterocyclic compounds. Hence, keeping these reports in view and continuation of our search for 3-aroyl prop-2-enoic acid derivatives[8-13], the present work deals with the study of behaviour of 3-(4- bromobenzoyl)prop-2-enoic acid towards the action of different nucleophilic species including carbon, nitrogen nucleophiles and the utility some of the reaction products in heterocyclic synthesis, hoping to get new compounds of anticipated biological activities.
2. Results & Discussion
- Addition of ethyl cyanoacetate on 3-(4-bromobenzoyl)- acrylic acid (1) in the presence of ammonium acetate yielded a mixture of ethyl-2-amino-4-carboxy-6-(4-bromophenyl)- nicotinate (2) and 2-oxo-3-cyano--6-(4-bromophenyl)- pyridine-4-carboxylic acid (3), whereas the compounds 2 and 3 can be explained[14]. Also, in the present investigation, similar treatment of 1 with malononitrile in the presence of ammonium acetate in boiling butanol gave 2-amino-3- cyano-6-(4-bromo)phenyl 3,4-dihydropyridine 4-carboxylic acid (4). The structure of 1:1 adduct 4 obtained by base catalyzed Michael addition of malononitrile to acid 1 is elucidated by studying their spectroscopic properties. The mass spectrum of 4 shows the correct molecular ion peak. On the other hand,when compound 1 was treated with malon- onitrile in the presence of piperidine in boiling ethanol yielded 2-amino-3-cyano-6-(4-bromophenyl)-pyrane 4-car- boxylic acid (5). (Scheme 1).
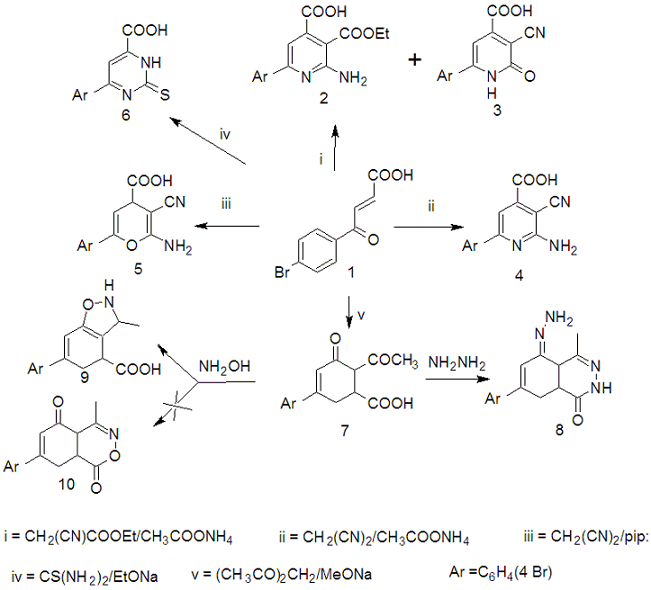 | Scheme 1. |
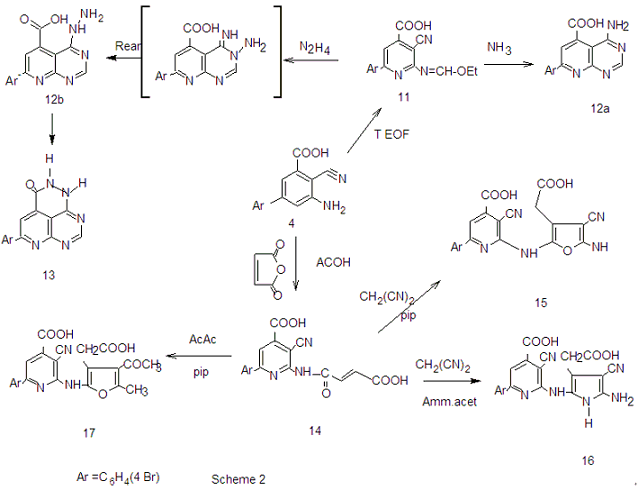 | Scheme 2. |
 , C=
, C= , C
, C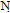 . OH. and showed no absorption frequency in the NH region. The H-NMR spectrum of compound 11 showed singlet signal of the azamethine proton(N=CH) at δ 6.76,triplet at δ 1.1 and quartet signal at δ 4.3 assigned for (OCH2CH3).Ammonolysis of compound 11 gave 4-amino7-(4-bromophenyl) pyridine [2,3-d] pyrimidine-5-carboxylic acid 12.structure of 12 was inferred from correct analytical data and H-NMR exhibited exchangeable singlet at 8.23 assigned for (NH2) at the pyrimidine ring and broad exchangeable singlet at 11.6 correlated with (COOH) proton at the pyridine ring. The reaction involved nucleophilic substitution on the unsaturated carbon atom followed by ring closure to afford 12. Inteaction of compound 11 with hydrazine hydrate[32] in boiling ethanol yielded 5-(4-bromophenyl)-1,2,3-trihydro- 1,2,6,7,9-pentaza-phenalen-3(3H)-one 13, without isolation the hydrazino derivative 12b. This could be explained by the formation of the imino derivative first, which the presence of a base (hydrazine hydrate) underwent a Dimroth rearrangement[33-35] to give the thermodynamically more stable hydrazine derivative which underwent ring closure and yielded the desired product 13. IR spectrum of compound 13 exhibited strong absorption bands at 1615,1655and 3325 cm-1 corresponding to C=N, νC= O (amide) and devoid νNH any band at 2220.The H-NMR spectrum revealed exchangeable two singlet signals at 9.35 and 5.21 ppm attributable to the protons of (CONH) and(CONHNH) at the pyridazinone ring,and devoid any exchangeable (OH).The N-cyclic maleamic acid 14[36,37] has been synthesized via interaction of 4 with maleic anhydride in refluxing acetic acid (Scheme-2).The N-cyclic maleamic acid was constructed such that due to the ring-cleaved structure of maleic anhydride (-COCH=CH-COOH) that bonded to the amino group (NH2) of the starting N-cyclic amine via a maleamide bond (-NH-CO-). The structure of the N-cyclic maleamic acid 14 has confirmed by analytical and spectral analyses. IR spectrum of compound 14 exhibited strong absorption bands at 1634, 1719,2209,3217,3347 and 3445 cm-1 attributable to νC=O (amide), νC=O (carboxyl), νCN, νNH and νOH respectively. Interaction of the compound 14 with malononitrile in the presence of piperidine as catalyst afforded 2-[5-amino-3-(carboxymethyl)-4-cyano-furan-2-ylamino]-6-(4-bromophenyl)-3- cyano-pyridine-4-carboxylic acid 15. lR spectrum of compound 15 revealed strong absorption bands at 1629,1719,2207,3220,3372 and 3447 due to νC=N, νC= O, νCN and νNH and/or νOH respectively. Refluxing compound 14 with malononitrile in the presence of ammonium acetate [38] afforded 2- [5-amino-3-( carboxymethyl)-4-cyano-lH-pyrrol-2-ylamino)]-6-( 4-bromophenyl)- 3-cyano-pyridine-4-carboxylic acid 16. The reactions take place via Michael addition followed by ring closure to give the desire products 15,16 respectively. Structure of compound 16 was inferred from microanalytical and spectral data.Its IR spectrum revealed strong absorption bands at 1632, 1720, 2210, 3217, 3354 and 3451 cm-1 attributable to. νC=N, νC=O, νCN, νNH and/or νOH respectively. The IH-NMRspectrum showed exchangeable (NH2) as well as singlet consistent with protons of methylene group of (CH2COOH). When compound 14 was allowed to react with acetylacetone in the presence of piperidine as catalyst, it yielded 2-[4-acetyl-3-carboxy methyl)-5-methyl furan-2-yl amino]-6-(4-bromophenyl)- 3- cyanopyridine- 4- carboxylic acid 17. The IR spectrum of 17 revealed strong absorption bands at 1631, 1720,2210,3213,3347 and 3448 cm-1 attributable to νC=N, νC=O, νCN, νNH, and νOH respectively. The IH-NMR spectrum exhibited absorption signals correlated with the (CH3), (COCH3) and (CH2COOH) protons. El-MS exhibited m/z 455 and 453 corresponding to ([M+2] and M -C02) respectively.
. OH. and showed no absorption frequency in the NH region. The H-NMR spectrum of compound 11 showed singlet signal of the azamethine proton(N=CH) at δ 6.76,triplet at δ 1.1 and quartet signal at δ 4.3 assigned for (OCH2CH3).Ammonolysis of compound 11 gave 4-amino7-(4-bromophenyl) pyridine [2,3-d] pyrimidine-5-carboxylic acid 12.structure of 12 was inferred from correct analytical data and H-NMR exhibited exchangeable singlet at 8.23 assigned for (NH2) at the pyrimidine ring and broad exchangeable singlet at 11.6 correlated with (COOH) proton at the pyridine ring. The reaction involved nucleophilic substitution on the unsaturated carbon atom followed by ring closure to afford 12. Inteaction of compound 11 with hydrazine hydrate[32] in boiling ethanol yielded 5-(4-bromophenyl)-1,2,3-trihydro- 1,2,6,7,9-pentaza-phenalen-3(3H)-one 13, without isolation the hydrazino derivative 12b. This could be explained by the formation of the imino derivative first, which the presence of a base (hydrazine hydrate) underwent a Dimroth rearrangement[33-35] to give the thermodynamically more stable hydrazine derivative which underwent ring closure and yielded the desired product 13. IR spectrum of compound 13 exhibited strong absorption bands at 1615,1655and 3325 cm-1 corresponding to C=N, νC= O (amide) and devoid νNH any band at 2220.The H-NMR spectrum revealed exchangeable two singlet signals at 9.35 and 5.21 ppm attributable to the protons of (CONH) and(CONHNH) at the pyridazinone ring,and devoid any exchangeable (OH).The N-cyclic maleamic acid 14[36,37] has been synthesized via interaction of 4 with maleic anhydride in refluxing acetic acid (Scheme-2).The N-cyclic maleamic acid was constructed such that due to the ring-cleaved structure of maleic anhydride (-COCH=CH-COOH) that bonded to the amino group (NH2) of the starting N-cyclic amine via a maleamide bond (-NH-CO-). The structure of the N-cyclic maleamic acid 14 has confirmed by analytical and spectral analyses. IR spectrum of compound 14 exhibited strong absorption bands at 1634, 1719,2209,3217,3347 and 3445 cm-1 attributable to νC=O (amide), νC=O (carboxyl), νCN, νNH and νOH respectively. Interaction of the compound 14 with malononitrile in the presence of piperidine as catalyst afforded 2-[5-amino-3-(carboxymethyl)-4-cyano-furan-2-ylamino]-6-(4-bromophenyl)-3- cyano-pyridine-4-carboxylic acid 15. lR spectrum of compound 15 revealed strong absorption bands at 1629,1719,2207,3220,3372 and 3447 due to νC=N, νC= O, νCN and νNH and/or νOH respectively. Refluxing compound 14 with malononitrile in the presence of ammonium acetate [38] afforded 2- [5-amino-3-( carboxymethyl)-4-cyano-lH-pyrrol-2-ylamino)]-6-( 4-bromophenyl)- 3-cyano-pyridine-4-carboxylic acid 16. The reactions take place via Michael addition followed by ring closure to give the desire products 15,16 respectively. Structure of compound 16 was inferred from microanalytical and spectral data.Its IR spectrum revealed strong absorption bands at 1632, 1720, 2210, 3217, 3354 and 3451 cm-1 attributable to. νC=N, νC=O, νCN, νNH and/or νOH respectively. The IH-NMRspectrum showed exchangeable (NH2) as well as singlet consistent with protons of methylene group of (CH2COOH). When compound 14 was allowed to react with acetylacetone in the presence of piperidine as catalyst, it yielded 2-[4-acetyl-3-carboxy methyl)-5-methyl furan-2-yl amino]-6-(4-bromophenyl)- 3- cyanopyridine- 4- carboxylic acid 17. The IR spectrum of 17 revealed strong absorption bands at 1631, 1720,2210,3213,3347 and 3448 cm-1 attributable to νC=N, νC=O, νCN, νNH, and νOH respectively. The IH-NMR spectrum exhibited absorption signals correlated with the (CH3), (COCH3) and (CH2COOH) protons. El-MS exhibited m/z 455 and 453 corresponding to ([M+2] and M -C02) respectively.3. Conclusions
- The proposed procedure is an easy and inexpensive methodology for the synthesized compounds. New some interesting heterocycles were synthesized by the reaction of 3-(4-bromo benzoyl)prop-2-enoic acid precursors with ethyl cyano acetate,malononitrile in different condition,acetyl acetone.Synthesis a various substituted pyridines derivatives incorporated with pyrimidine 12,13 and furan 15,17 precursors to enhance their biological activity .Finally, given the structures by spectral data (IR,H-NMR,MS
4. Experimental
- All melting points are uncorrected. Elemental analyses were carried out in the Microanalytical Center, the center publication for research, Cairo, Egypt. By Elementar Viro El Microanalysis IR spectra (KBr) were recorded on infrared spectrometer ST-IR DOMEM Hartman Braun, Model: MBB 157, Canada and H-NMR spectra recorded on a varian 300 MHz (Germany 1999) using TMS as internal standard. The mass spectra were recorded on Shimadzu GCMS-QP-1000 EX mass spectrometer at 70e.v. homogeneity of all compounds synthesized was checked by TLC.Ethyl-2-amino-4-carboxy-6-(4-bromo phenyl) nicotinate (2)A solution of
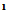 (2.5 g,0.01 mol) and 3mL ethylcyanoacetate and 5 g ammonium acetate was heated in water bath for 3h, then poured water the solid that separated with crystallized form ethanol afford 2. M.wt 365( C15H13BrN2O4 ) (m.p.115℃, yield 54%, % calcd/found[(C 49.30/49.22,%H 3.5/3.46, % N 7.67/7.66, % Br 21.9/21.6]. IR NH.3437, C=O (acid and ester) 1686,1733, C=N 1620 cm-1 1HNMR 1.3(t, 3H, J=7.4), 3.9(s, 2H, NH2), 4.05(q, 2H, J=7.4), 7.57.8 (m, 5H, ArH), 11.1(s, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 366 and 364 corresponding to (M+2).+ (M.+).respectively. 3-cyano-4-carboxy-6-(4-bromo phenyl) 2(1H)-pyridones (3)A solution of
(2.5 g,0.01 mol) and 3mL ethylcyanoacetate and 5 g ammonium acetate was heated in water bath for 3h, then poured water the solid that separated with crystallized form ethanol afford 2. M.wt 365( C15H13BrN2O4 ) (m.p.115℃, yield 54%, % calcd/found[(C 49.30/49.22,%H 3.5/3.46, % N 7.67/7.66, % Br 21.9/21.6]. IR NH.3437, C=O (acid and ester) 1686,1733, C=N 1620 cm-1 1HNMR 1.3(t, 3H, J=7.4), 3.9(s, 2H, NH2), 4.05(q, 2H, J=7.4), 7.57.8 (m, 5H, ArH), 11.1(s, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 366 and 364 corresponding to (M+2).+ (M.+).respectively. 3-cyano-4-carboxy-6-(4-bromo phenyl) 2(1H)-pyridones (3)A solution of 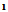 (2.5 g,0.01 mol) and 3mL ethylcyanoacetate and 5 g ammonium acetate was heated in water bath for 3hs, then poured water the solid that separated was crystallized form Benzene -Ethanol afford 3. M.wt=319(C13H7 Br N2O3) (m.p 160℃, yield 40%, % calcd/found[(C 48.92/ 48.94, H 2.19/2.18, N 8.78/8.81, Br 25.07/25.11 IR NH 3354, CN 2212, max of two carbonyl groups (cyclic amide and carboxyl group), 1655, 1678, and C=N1628, 1HNMR 6.8-7.5(m, 5H, ArH), 10.03(s, 1H, NH) 12.1(s, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 320 and 318 corresponding to (M+2).+ (M.+).respectively. 2-amino-3-cyano-4-carboxy-6-(4-bromophenyl)-3,4-dihydropyridine (4)A solution of
(2.5 g,0.01 mol) and 3mL ethylcyanoacetate and 5 g ammonium acetate was heated in water bath for 3hs, then poured water the solid that separated was crystallized form Benzene -Ethanol afford 3. M.wt=319(C13H7 Br N2O3) (m.p 160℃, yield 40%, % calcd/found[(C 48.92/ 48.94, H 2.19/2.18, N 8.78/8.81, Br 25.07/25.11 IR NH 3354, CN 2212, max of two carbonyl groups (cyclic amide and carboxyl group), 1655, 1678, and C=N1628, 1HNMR 6.8-7.5(m, 5H, ArH), 10.03(s, 1H, NH) 12.1(s, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 320 and 318 corresponding to (M+2).+ (M.+).respectively. 2-amino-3-cyano-4-carboxy-6-(4-bromophenyl)-3,4-dihydropyridine (4)A solution of 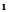 (2,5 g,0.01 mol) in n-butanol (20 mL) was treated with malononitrile (0.7 g,0.01 mole) in 5 g ammonium acetate refluxed for 3 h, then poured water with heating to replaceable n-butanol by water, then take the filtrate with ice/HCl. The solid that separated on cooling was crystallized form ethanol afford 4.M.wt=320 (C13H10BrN3O2) m.p. 220℃, yield 75% calcd/found[(C48. 75/49.00, H 3.13/3.22, N 13.12/13.02, Br25.00/25.08] IR OH, NH, CN, C=O at 3422, 3220, 2211, 1707 cm-1 1HNMR 2.4(s, 2H, NH2), 2.8(d, 1H, CHCN, J=8.5), 3.2(dd, 1H, CHCO.5, J=8.5, J=6.4), 5.6(d, 1H, H-5 pyr, J=6.4) 7.4-7.5(m, 4H, Ar-H), 11.03(s, 1H, exchangeable proton The EI-MS shows the molecular ion peak at m/e 321 and 319 corresponding to (M+2).+ (M.+). respectively 2-amino-3-cyano-4-carboxy-6-(4-bromophenyl)pyran(5)A solution of
(2,5 g,0.01 mol) in n-butanol (20 mL) was treated with malononitrile (0.7 g,0.01 mole) in 5 g ammonium acetate refluxed for 3 h, then poured water with heating to replaceable n-butanol by water, then take the filtrate with ice/HCl. The solid that separated on cooling was crystallized form ethanol afford 4.M.wt=320 (C13H10BrN3O2) m.p. 220℃, yield 75% calcd/found[(C48. 75/49.00, H 3.13/3.22, N 13.12/13.02, Br25.00/25.08] IR OH, NH, CN, C=O at 3422, 3220, 2211, 1707 cm-1 1HNMR 2.4(s, 2H, NH2), 2.8(d, 1H, CHCN, J=8.5), 3.2(dd, 1H, CHCO.5, J=8.5, J=6.4), 5.6(d, 1H, H-5 pyr, J=6.4) 7.4-7.5(m, 4H, Ar-H), 11.03(s, 1H, exchangeable proton The EI-MS shows the molecular ion peak at m/e 321 and 319 corresponding to (M+2).+ (M.+). respectively 2-amino-3-cyano-4-carboxy-6-(4-bromophenyl)pyran(5)A solution of 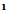 (2.5 g,0.01 mol) in ethanol (100 mL) was treated with malononitrile (0.7 g,0.01 mol) in piperidine (2mL). Stirred at room temperature for 1h, then concentrated the solution and poured H2O/HCL. The solid that separated on cooling was crystallized form ethanol afford 5. M.wt= 321(C13H9BrN2O3)(m.p 121℃, yield 70%, % calcd/found[(C 48.59/48.48, H 2.80/2.77, N 8.72/8.68, Br 24.92/25.81 IR N
(2.5 g,0.01 mol) in ethanol (100 mL) was treated with malononitrile (0.7 g,0.01 mol) in piperidine (2mL). Stirred at room temperature for 1h, then concentrated the solution and poured H2O/HCL. The solid that separated on cooling was crystallized form ethanol afford 5. M.wt= 321(C13H9BrN2O3)(m.p 121℃, yield 70%, % calcd/found[(C 48.59/48.48, H 2.80/2.77, N 8.72/8.68, Br 24.92/25.81 IR N 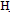 (bonded and non bonded), 3227, 2339, C=O1705cm-1 and C=N1625, 1HNMR 2.6(s, 2H, NH2), 2.8(d, 1H, CH-CN),3,2(d,1H,CH-CO),7.-7.5(m,5H,Ar-H),11.03(s,1H,exchangeable proton) . The EI-MS shows the molecular ion peak at m/e 322 and 320 corresponding to (M+2).+ (M.+).respectively 4-(4-bromophenyl)-6-carboxy pyrimidin-2(1H)-thione (6)A solution of
(bonded and non bonded), 3227, 2339, C=O1705cm-1 and C=N1625, 1HNMR 2.6(s, 2H, NH2), 2.8(d, 1H, CH-CN),3,2(d,1H,CH-CO),7.-7.5(m,5H,Ar-H),11.03(s,1H,exchangeable proton) . The EI-MS shows the molecular ion peak at m/e 322 and 320 corresponding to (M+2).+ (M.+).respectively 4-(4-bromophenyl)-6-carboxy pyrimidin-2(1H)-thione (6)A solution of 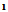 (2.5 g,0.01 mol) in 0.5 g sodium and 15 mL ethanol was treated with thiourea (0,76 g,0.01 mol) refluxed for 4h. The solid that separated after cooling was crystallized form the suitable solvent afford 6 .M.wt=311(C11H7 Br N2O2 S)(m.p 200℃, yield 75% , % calcd/found[(C 42.44/42.48,H 2.25/2.35,N 8.99/8.98,S 10.28/10.23, Br 25.72/25.61. IR OH and/or N
(2.5 g,0.01 mol) in 0.5 g sodium and 15 mL ethanol was treated with thiourea (0,76 g,0.01 mol) refluxed for 4h. The solid that separated after cooling was crystallized form the suitable solvent afford 6 .M.wt=311(C11H7 Br N2O2 S)(m.p 200℃, yield 75% , % calcd/found[(C 42.44/42.48,H 2.25/2.35,N 8.99/8.98,S 10.28/10.23, Br 25.72/25.61. IR OH and/or N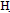 3379, 3275, 3180 and C=O 1676 C=N at 1613cm-1,1HNMR 3.9(s,1H,NH),6.4(s,1H,pyrimidine proton),7.4(d,4H.Ar-H),11(s,1H,COOH). The EI-MS shows the molecular ion peak at m/e 312 and 310 corresponding to (M+2).+ (M.+).respectively 3-(4-bromophenyl)-5-carboxy-6-acetylcyclohexen-1-one (7)A solution of
3379, 3275, 3180 and C=O 1676 C=N at 1613cm-1,1HNMR 3.9(s,1H,NH),6.4(s,1H,pyrimidine proton),7.4(d,4H.Ar-H),11(s,1H,COOH). The EI-MS shows the molecular ion peak at m/e 312 and 310 corresponding to (M+2).+ (M.+).respectively 3-(4-bromophenyl)-5-carboxy-6-acetylcyclohexen-1-one (7)A solution of 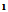 (2.5 g,0.01 mole) in 30 mL ethanol was treated with acetyl acetone (0.01 mole) refluxed in water bath for 4h, then poured water. The solid that separated on cooling was crystallized form the suitable solvent afford 7. M.wt=337(C15H13BrO4) (m.p 119℃, yield 75%, % calcd/ found[(C53.41/53.60,H 3.85/4.00, Br 23.72/23.72 IR exhibits O
(2.5 g,0.01 mole) in 30 mL ethanol was treated with acetyl acetone (0.01 mole) refluxed in water bath for 4h, then poured water. The solid that separated on cooling was crystallized form the suitable solvent afford 7. M.wt=337(C15H13BrO4) (m.p 119℃, yield 75%, % calcd/ found[(C53.41/53.60,H 3.85/4.00, Br 23.72/23.72 IR exhibits O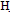 = 3430, C
= 3430, C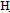 ar = 3030 CH ali = 2888, C = O1687,1695cm-1, 1HNMR 2.2(s,3H.CH3CO), 2.3(dd,2H, diasteriotopic protons of allylic cyclohexanone), 2.9(m, 1H, CH-COO), 3.6(d d,1H,CH-CO), 6.8-7.3(m,5H,Ar-H), 11.3(s, 1H, COOH). The EI-MS spectrum that the m/e 319,317 (M.+- H2O). 1-methyl 4,5-dihydro-6-(4-bromo phenyl) 8-hydrazino phthalazin-4(3H)-one (8)A solution of (3.4 g,0.01 mol) in 50 mL ethanol was treated with hydrazine hydrae (0.01 mole) refluxed in for 3h, then heated to concentrate. The solid that separated after cooling was crystallized form the suitable solvent afford 8. M.wt=347(C15H15 Br N4O (m.p 280℃, yield 55%, % calcd/found[(C 51.87/51.60,H 4.32/4.30,N 16.13/16.11 Br 23.05/23.12 IR (N H, 3200 3262 bonded and nonbonded C=O1657cm-1.1HNMR 0.9(S,3H,CH3),2.1(s,2H,N=NH2), 2.5(d,2H,allylic,J=8.7), 3.1(dt,1Hb,fused ring, J=8.7, J=9.2), 3.5(d,1Ha,fused-ringJ=9.2), 6.4(s,1H,olefin proton), 7.27.4 (dd,4H,ArH), 11(s,1H,NH). EI-MS exhibits molecular ion peak m/e (348, 18.2%) beside some of abundant peaks. 3- methyl 6(4-bromo phenyl)2,3-dihydro-1,2- benzoxazole-4-carboxylic acid (9)A solution of 7 (3.4 g,0.01 mol) in 20 mL pyridine was treated with hydroxylamine (0.01 mole) refluxed for 3hs, then poured ice/H2O. The solid that separated after cooling was crystallized form Benzene to afford 9. M.wt=336(C15H14 Br NO3 (m.p 166℃, yield 55%, % calcd/found[(C 53.57/53.60,H 4.16/4.20,N 4.16/4.21 Br 23.81/23.77 IR (OH 3250(saturatedacid), C=O, 1700, C=N1618cm.1HNMR 1.2 (d,3H,CH3,J=7.5) 2.1(s,2H,N=), 2.3(dd,2H,CH2 diasteriotopicprotons J=14.2, J=4.3 & J=9.4, J=4.3), 2.9(dd,1H, CHCOO, J=14.2, J=9.4), 4(q,1H,CH-N,J=7.5), 7.27.5(m,5H, ArH), 8.2(s,1H,NH), 11(s,1H,COOH). The EI-MS shows the molecular ion peak at m/e 337 and 335 corresponding to (M+2).+ (M.+).respectively 6-(4-bromo phenyl)-3-cyano-2-(ethoxymethyleneamino)-pyridine-4-carboxylic acid (11)Pyridine derivative 4 (3.2 g,0.01 mol ) in (0.35 mL, 0.01mol triethylorthoformate was stirred under reflux for 5 h. The reaction mixture was concentrated and the obtained brown precipitate then crystallized from ethanol/water to afford ethoxy methylene amino pyridine 11. M.wt=376 (C16H13BrN2O4(m.p 175℃, yield 75%, % calcd/found [(C51.07/51.09, H 4.16/4.20,N 7.44/7.41 Br 21.27/21.27 IR (OH3350(saturated acid), CN 2200 C=O,1718, C=N1628cm 1HNMR 1.33 (t, 3H, OCH2CH3, J=7.6), 2.7(d, 1H, CHCN J=8.5), 3.1(dd,1H,CHCO,J=8.5,J=6.4) 4.29(q,2H, OCH2, J=7.6), 5.16(d, 1H, J=6.4 H-5 Pyr), 7.5-7.7(m, 4H, ArH)- 8.2(s,1H,N=CH),11.5(s,1H,OH,exchangeable proton) The EI-MS shows the molecular ion peak at m/e 377 and 375 corresponding to (M+2).+ (M.+).respectively 4-amino-7-(4-bromophenyl)-5H-pyridino[2,3-d]pyrimidine-5-carboxylic acid (12a)A solution of ethoxy methylene amino-4H-pyridine 11 (3.8 g,0.01mol) and absolute ethanol (10 mL),ammonia solution (0.015mL,0.01mol) was added. The resulting mixture was refluxed for 2 h.After cooling, the reaction mixture was acidified with very diluted solution of cold HCl, the precipitate formed was formed was filtered off and washed on the filter funnel with water,dried then crystallized from ethanol/water to afford the amino pyrido-pyrimidine(12) M.wt=347(C14H10BrN3O3(m.p 140℃, yield 85%, % calcd/ found[(C48.41/48.60,H 2.88/2.80,N 12.10/12.21 Br 23.05/ 23.12 IR, (OH(NH)3359, C=O,1730, C=N1648cm. 1HNMR, 2.9(d,1H,CHC-NH2 J=8.1), 3.1(dd,1H,CHCO,J=8.1,J=6.4) 5.2(d,1H.H-5pyr,J=6.4), 7.3(s,2H,exchangeable NH2), 7.6- 7.5(m,4H,ArH) 9.2(s,1H,CH,pyrimidine), 11.45(S,1H, exchangeable OH The EI-MS shows the molecular ion peak at m/e 348 and 346 corresponding to (M+2).+ (M.+).respectively 5-(4-bromophenyl)-1,2,3a-trihydro-6-oxa-1,2,,7,9-tetraphenalen-3(3H)-one (13).A mixture of 11 (3.8 g,0.01mol) and hydrazine hydrate [34] (0.012 mL,0.01mol) in absolute ethanol (30ml) was refluxed for 7 h . The reaction mixture was left to cool at r.t.then acidified with diluted HCl ,the formed solid was filtered off , washed with cold water, dried and crystallized from the proper solvent to afford pyridopyrimidine(13) M.wt=344 (C14H12 Br N4O2 (m.p 136℃, yield 85%, % calcd/found[(C 48.83/48.62,H 3.48/3.51 ,N16.26/16.21, Br23.25/23.27. IR(NH3320,C=O1650, C=N1615cm.1HNMR,2.3(d, 1H, CHC-NH J=8.5),3.1(dd, 1H, CHCO, J=8.5, J=6.4), 4.3(s,1H,CONH(NH)exchangeable), 4.9(d, 1H,H-5 pyr, J=6.4), 7.2-7.5(m, 4H, ArH)8.7(s, 1H, CONH), 9.4(s,1H,CH pyrimidine). The EI-MS shows the molecular ion peak at m/e 345 and 343 corresponding to (M+2).+ (M.+).respectively 2-(3-carboxy acrylamido)-6-(4-bromo phenyl)-3- cyano- pyridine-4-carboxylic acid (14). Maleic anhydride (0.98 mg, 0.01 mol) was completely dissolved at room temperature in glacial acetic acid or THF (10 mL), and then pyridine derivative 4 (3.2 g,0.01 mol) was added to the solution; the resulting mixture was stirred under reflux for l h. The reaction mixture was allowed to cool at room temperature, then poured into water (20 ml), the precipitate formed was filtered off, washed with water,dried and crystallized from methanol to afford N-substituted maleamic acid 14. M.wt=416(C17H10 Br N3O5 (m.p 166℃, yield 55%, % calcd/found[(C 49.04/49.20,H 2.40/2.45,N 10.09/ 10.09 Br 19.23/19.37 IR, (OH and/or NH 3450-3372- 3225, CN 2210, C=O,1720, C=O amide 1648cm. 1HNMR 2.2.9(d,1H,CHCN J=8.5), 3.1(dd,1H,CHCO, J=8.5, J=6.4) 5.4(d,1H,H5- pyr,J=6.4)6.6(d,1H,J=15.3,CH=),7.15 (d,1H,J=15.3, =CH)7.57.7(m, 4H, ArH)10.2(S, 1H, NHCO), 11.7(s,1H,exchangeable OH),12.3(brs,1H,COOH). The EI- MS shows the molecular ion peak at m/e 317 and 315 corresponding to (M+2).+ (M.+).respectively 2-[5-amino-3-(carboxymetbyl)-4-cyanofuran- 2- ylamino 1-6-(4-bromophenyl)-3-cyano pyridine-4-carboxylic acid (15). To a solution of -cyclic maleamic acid 14 (2.00 g, 5.2 mmol) and malononitrile (0.34 g, 5.2 mmol) in DMF (4 mL) few drops of piperidine was added; the resulting mixture was refluxed at 60 C for 5 h. The reaction mixture was allowed to cool at room temperature then acidified with diluted acetic acid 20 mL, the solid formed was filtered off, washed with water, dried and crystallized from methanol/water to afford furan derivative 15 M.wt=482(C20H12 Br N5O5 (m.p 166℃, yield 75%, % calcd/fou[(C 49.79/49.60, H 2.48/2.40, N 14.52/14.41 Br 16.59/16.77 IR (OH and/or NH) 3250,3370, 3445., CN 2210., C=O,1720, C=N1628cm.1HNMR 2.9(d, 1H, CHCN J=8.5), 3.1(dd, 1H, CHCO, J=8.5, J=6.4) 3.83(s, 2H,CH2) 5.9(d, 1H, H-5 pyr, J=6.4), 7.2-7.5(m, 4H, ArH), 8.2(S, 2H, NH2),10.5(s,1H,exchangeableNH) 12.3(brs, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 483 and 481corresponding to (M+2).+ (M.+).respectively 2-[5-amino-3-(carboxymethyl)-4-cyano-lH-pyrrol-2-ylamino)]-6(4-bromophenyl)-3-cyanopyridine-4-carboxylic acid (16) A mixture ofN-cyclic maleamic acid 14 (2 g, 5.2 mmol) and malononitrile[36] (0.34 g, 5.2 mmol) in DMF (3 mL) in the presence of ammonium acetate was refluxed at 60 C for 3 h. The reaction mixture was allowed to cool at room temperature then poured into diluted solution of acetic acid 20 ml, the solid formed was filtered off, washed with water, dried and crystallized from ethanol/water to afford aminopyrrole derivative 16 M.wt=481(C20H13BrN6O4 (m.p 166℃, yield 75%, % calcd/found[(C 49.81/49.70,H 2.70/2.64,N 17.46/17.41 Br 16.59/16.77 IR(OH and/ or NH) 3250,3350, 3440, CN 2218, C=O,1720, C=N1628cm. 1HNMR 2.8(d, 1H, CHCN J=8.5), 3.2(dd, 1H, CHCO, J=8.5, J=6.4) 3.8(s, 2H, CH2), 5.2(d, 1H, H-5 pyr)7.2-7.5(m, 4H, ArH) 8.4(s, 1H,exchangeable NH), 11.8(s,1H,exchangeable NH 12.3(s, 1H,COOH). The EI-MS shows the molecular ion peak at m/e 482 and 480 corresponding to (M+2).+ (M.+).respectively 2-[4-acetyl-3-(carboxymetbyl)-5-methylf uran-2-ylamino 1-6-(4-bromo phenyl)-3-cyanopyridine-4-carboxylic acid (17) A mixture of N-cyclic maleamic acid 14 (2 g, 5.2 mmol) and acetylacetone (0.53 mL, 5.2 mmol) in DMF (3 mL) in the presence of piperidine was refluxed at 60 C for 3 h. The reaction mixture was allowed to cool at room temperature then poured into diluted solution of acetic acid (200 mL), the solid formed was filtered off, washed with water, dried and crystallized from ethanol/ water to afford furan derivatives 17. M.wt=498(C22H16 Br N3O6 (m.p 166℃, yield 55%, % calcd/found[(C53.01/53.20,H 3.21/3.20,N 8.43/8.61 Br 16.06/16.21 IR(OHand/orNH) 3250,3330,3515 CN 2208, C=O,1720, C=N1620cm.1HNMR 2.6(s,3H,OCH3)2.9(d,1H, CHCN J=8.5),3.1(dd, 1H, CHCO, J=8.5, J=6.5), 3.8(s, 2H, CH2), 5.6(d,1H,H-5 pyr J=6.5), 7.2-7.5(m,4H,ArH), 8.2(s, 2H, NH2), 10.6(brs, 1H, exchangeable OH), 12.2(s, 1H, COOH). El-MS exhibited m/z 454 and 452 corresponding to ([M+2]and M -C02) respectively.
ar = 3030 CH ali = 2888, C = O1687,1695cm-1, 1HNMR 2.2(s,3H.CH3CO), 2.3(dd,2H, diasteriotopic protons of allylic cyclohexanone), 2.9(m, 1H, CH-COO), 3.6(d d,1H,CH-CO), 6.8-7.3(m,5H,Ar-H), 11.3(s, 1H, COOH). The EI-MS spectrum that the m/e 319,317 (M.+- H2O). 1-methyl 4,5-dihydro-6-(4-bromo phenyl) 8-hydrazino phthalazin-4(3H)-one (8)A solution of (3.4 g,0.01 mol) in 50 mL ethanol was treated with hydrazine hydrae (0.01 mole) refluxed in for 3h, then heated to concentrate. The solid that separated after cooling was crystallized form the suitable solvent afford 8. M.wt=347(C15H15 Br N4O (m.p 280℃, yield 55%, % calcd/found[(C 51.87/51.60,H 4.32/4.30,N 16.13/16.11 Br 23.05/23.12 IR (N H, 3200 3262 bonded and nonbonded C=O1657cm-1.1HNMR 0.9(S,3H,CH3),2.1(s,2H,N=NH2), 2.5(d,2H,allylic,J=8.7), 3.1(dt,1Hb,fused ring, J=8.7, J=9.2), 3.5(d,1Ha,fused-ringJ=9.2), 6.4(s,1H,olefin proton), 7.27.4 (dd,4H,ArH), 11(s,1H,NH). EI-MS exhibits molecular ion peak m/e (348, 18.2%) beside some of abundant peaks. 3- methyl 6(4-bromo phenyl)2,3-dihydro-1,2- benzoxazole-4-carboxylic acid (9)A solution of 7 (3.4 g,0.01 mol) in 20 mL pyridine was treated with hydroxylamine (0.01 mole) refluxed for 3hs, then poured ice/H2O. The solid that separated after cooling was crystallized form Benzene to afford 9. M.wt=336(C15H14 Br NO3 (m.p 166℃, yield 55%, % calcd/found[(C 53.57/53.60,H 4.16/4.20,N 4.16/4.21 Br 23.81/23.77 IR (OH 3250(saturatedacid), C=O, 1700, C=N1618cm.1HNMR 1.2 (d,3H,CH3,J=7.5) 2.1(s,2H,N=), 2.3(dd,2H,CH2 diasteriotopicprotons J=14.2, J=4.3 & J=9.4, J=4.3), 2.9(dd,1H, CHCOO, J=14.2, J=9.4), 4(q,1H,CH-N,J=7.5), 7.27.5(m,5H, ArH), 8.2(s,1H,NH), 11(s,1H,COOH). The EI-MS shows the molecular ion peak at m/e 337 and 335 corresponding to (M+2).+ (M.+).respectively 6-(4-bromo phenyl)-3-cyano-2-(ethoxymethyleneamino)-pyridine-4-carboxylic acid (11)Pyridine derivative 4 (3.2 g,0.01 mol ) in (0.35 mL, 0.01mol triethylorthoformate was stirred under reflux for 5 h. The reaction mixture was concentrated and the obtained brown precipitate then crystallized from ethanol/water to afford ethoxy methylene amino pyridine 11. M.wt=376 (C16H13BrN2O4(m.p 175℃, yield 75%, % calcd/found [(C51.07/51.09, H 4.16/4.20,N 7.44/7.41 Br 21.27/21.27 IR (OH3350(saturated acid), CN 2200 C=O,1718, C=N1628cm 1HNMR 1.33 (t, 3H, OCH2CH3, J=7.6), 2.7(d, 1H, CHCN J=8.5), 3.1(dd,1H,CHCO,J=8.5,J=6.4) 4.29(q,2H, OCH2, J=7.6), 5.16(d, 1H, J=6.4 H-5 Pyr), 7.5-7.7(m, 4H, ArH)- 8.2(s,1H,N=CH),11.5(s,1H,OH,exchangeable proton) The EI-MS shows the molecular ion peak at m/e 377 and 375 corresponding to (M+2).+ (M.+).respectively 4-amino-7-(4-bromophenyl)-5H-pyridino[2,3-d]pyrimidine-5-carboxylic acid (12a)A solution of ethoxy methylene amino-4H-pyridine 11 (3.8 g,0.01mol) and absolute ethanol (10 mL),ammonia solution (0.015mL,0.01mol) was added. The resulting mixture was refluxed for 2 h.After cooling, the reaction mixture was acidified with very diluted solution of cold HCl, the precipitate formed was formed was filtered off and washed on the filter funnel with water,dried then crystallized from ethanol/water to afford the amino pyrido-pyrimidine(12) M.wt=347(C14H10BrN3O3(m.p 140℃, yield 85%, % calcd/ found[(C48.41/48.60,H 2.88/2.80,N 12.10/12.21 Br 23.05/ 23.12 IR, (OH(NH)3359, C=O,1730, C=N1648cm. 1HNMR, 2.9(d,1H,CHC-NH2 J=8.1), 3.1(dd,1H,CHCO,J=8.1,J=6.4) 5.2(d,1H.H-5pyr,J=6.4), 7.3(s,2H,exchangeable NH2), 7.6- 7.5(m,4H,ArH) 9.2(s,1H,CH,pyrimidine), 11.45(S,1H, exchangeable OH The EI-MS shows the molecular ion peak at m/e 348 and 346 corresponding to (M+2).+ (M.+).respectively 5-(4-bromophenyl)-1,2,3a-trihydro-6-oxa-1,2,,7,9-tetraphenalen-3(3H)-one (13).A mixture of 11 (3.8 g,0.01mol) and hydrazine hydrate [34] (0.012 mL,0.01mol) in absolute ethanol (30ml) was refluxed for 7 h . The reaction mixture was left to cool at r.t.then acidified with diluted HCl ,the formed solid was filtered off , washed with cold water, dried and crystallized from the proper solvent to afford pyridopyrimidine(13) M.wt=344 (C14H12 Br N4O2 (m.p 136℃, yield 85%, % calcd/found[(C 48.83/48.62,H 3.48/3.51 ,N16.26/16.21, Br23.25/23.27. IR(NH3320,C=O1650, C=N1615cm.1HNMR,2.3(d, 1H, CHC-NH J=8.5),3.1(dd, 1H, CHCO, J=8.5, J=6.4), 4.3(s,1H,CONH(NH)exchangeable), 4.9(d, 1H,H-5 pyr, J=6.4), 7.2-7.5(m, 4H, ArH)8.7(s, 1H, CONH), 9.4(s,1H,CH pyrimidine). The EI-MS shows the molecular ion peak at m/e 345 and 343 corresponding to (M+2).+ (M.+).respectively 2-(3-carboxy acrylamido)-6-(4-bromo phenyl)-3- cyano- pyridine-4-carboxylic acid (14). Maleic anhydride (0.98 mg, 0.01 mol) was completely dissolved at room temperature in glacial acetic acid or THF (10 mL), and then pyridine derivative 4 (3.2 g,0.01 mol) was added to the solution; the resulting mixture was stirred under reflux for l h. The reaction mixture was allowed to cool at room temperature, then poured into water (20 ml), the precipitate formed was filtered off, washed with water,dried and crystallized from methanol to afford N-substituted maleamic acid 14. M.wt=416(C17H10 Br N3O5 (m.p 166℃, yield 55%, % calcd/found[(C 49.04/49.20,H 2.40/2.45,N 10.09/ 10.09 Br 19.23/19.37 IR, (OH and/or NH 3450-3372- 3225, CN 2210, C=O,1720, C=O amide 1648cm. 1HNMR 2.2.9(d,1H,CHCN J=8.5), 3.1(dd,1H,CHCO, J=8.5, J=6.4) 5.4(d,1H,H5- pyr,J=6.4)6.6(d,1H,J=15.3,CH=),7.15 (d,1H,J=15.3, =CH)7.57.7(m, 4H, ArH)10.2(S, 1H, NHCO), 11.7(s,1H,exchangeable OH),12.3(brs,1H,COOH). The EI- MS shows the molecular ion peak at m/e 317 and 315 corresponding to (M+2).+ (M.+).respectively 2-[5-amino-3-(carboxymetbyl)-4-cyanofuran- 2- ylamino 1-6-(4-bromophenyl)-3-cyano pyridine-4-carboxylic acid (15). To a solution of -cyclic maleamic acid 14 (2.00 g, 5.2 mmol) and malononitrile (0.34 g, 5.2 mmol) in DMF (4 mL) few drops of piperidine was added; the resulting mixture was refluxed at 60 C for 5 h. The reaction mixture was allowed to cool at room temperature then acidified with diluted acetic acid 20 mL, the solid formed was filtered off, washed with water, dried and crystallized from methanol/water to afford furan derivative 15 M.wt=482(C20H12 Br N5O5 (m.p 166℃, yield 75%, % calcd/fou[(C 49.79/49.60, H 2.48/2.40, N 14.52/14.41 Br 16.59/16.77 IR (OH and/or NH) 3250,3370, 3445., CN 2210., C=O,1720, C=N1628cm.1HNMR 2.9(d, 1H, CHCN J=8.5), 3.1(dd, 1H, CHCO, J=8.5, J=6.4) 3.83(s, 2H,CH2) 5.9(d, 1H, H-5 pyr, J=6.4), 7.2-7.5(m, 4H, ArH), 8.2(S, 2H, NH2),10.5(s,1H,exchangeableNH) 12.3(brs, 1H, COOH). The EI-MS shows the molecular ion peak at m/e 483 and 481corresponding to (M+2).+ (M.+).respectively 2-[5-amino-3-(carboxymethyl)-4-cyano-lH-pyrrol-2-ylamino)]-6(4-bromophenyl)-3-cyanopyridine-4-carboxylic acid (16) A mixture ofN-cyclic maleamic acid 14 (2 g, 5.2 mmol) and malononitrile[36] (0.34 g, 5.2 mmol) in DMF (3 mL) in the presence of ammonium acetate was refluxed at 60 C for 3 h. The reaction mixture was allowed to cool at room temperature then poured into diluted solution of acetic acid 20 ml, the solid formed was filtered off, washed with water, dried and crystallized from ethanol/water to afford aminopyrrole derivative 16 M.wt=481(C20H13BrN6O4 (m.p 166℃, yield 75%, % calcd/found[(C 49.81/49.70,H 2.70/2.64,N 17.46/17.41 Br 16.59/16.77 IR(OH and/ or NH) 3250,3350, 3440, CN 2218, C=O,1720, C=N1628cm. 1HNMR 2.8(d, 1H, CHCN J=8.5), 3.2(dd, 1H, CHCO, J=8.5, J=6.4) 3.8(s, 2H, CH2), 5.2(d, 1H, H-5 pyr)7.2-7.5(m, 4H, ArH) 8.4(s, 1H,exchangeable NH), 11.8(s,1H,exchangeable NH 12.3(s, 1H,COOH). The EI-MS shows the molecular ion peak at m/e 482 and 480 corresponding to (M+2).+ (M.+).respectively 2-[4-acetyl-3-(carboxymetbyl)-5-methylf uran-2-ylamino 1-6-(4-bromo phenyl)-3-cyanopyridine-4-carboxylic acid (17) A mixture of N-cyclic maleamic acid 14 (2 g, 5.2 mmol) and acetylacetone (0.53 mL, 5.2 mmol) in DMF (3 mL) in the presence of piperidine was refluxed at 60 C for 3 h. The reaction mixture was allowed to cool at room temperature then poured into diluted solution of acetic acid (200 mL), the solid formed was filtered off, washed with water, dried and crystallized from ethanol/ water to afford furan derivatives 17. M.wt=498(C22H16 Br N3O6 (m.p 166℃, yield 55%, % calcd/found[(C53.01/53.20,H 3.21/3.20,N 8.43/8.61 Br 16.06/16.21 IR(OHand/orNH) 3250,3330,3515 CN 2208, C=O,1720, C=N1620cm.1HNMR 2.6(s,3H,OCH3)2.9(d,1H, CHCN J=8.5),3.1(dd, 1H, CHCO, J=8.5, J=6.5), 3.8(s, 2H, CH2), 5.6(d,1H,H-5 pyr J=6.5), 7.2-7.5(m,4H,ArH), 8.2(s, 2H, NH2), 10.6(brs, 1H, exchangeable OH), 12.2(s, 1H, COOH). El-MS exhibited m/z 454 and 452 corresponding to ([M+2]and M -C02) respectively.ACKNOWLEDGEMENTS
- Thanks for god to complete this research, the authors grateful to your collage in organic lab, Ain shams university and micro analytical data in Cairo University for providing facilities to carry out the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML