-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(2): 65-71
doi: 10.5923/j.chemistry.20110102.14
Utility of E-1-(4-Acetamidobenzoyl)-2-Oxirane Carboxylic Acid in Synthesis Some Fused Heterocycles and Spiro Compounds
Sameh. A. Rizk
Department of Chemistry, Faculty of Science, University of Ain Shams ,Cairo, Egypt
Correspondence to: Sameh. A. Rizk , Department of Chemistry, Faculty of Science, University of Ain Shams ,Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present work deals with the reaction of 4-(4-Acetyl amino phenyl-4-oxobut-2-enoic acid (1) with hydrogen peroxide afforded oxirane derivative 2.The latter compound was treated with 2-amino-5-aryl-1,3,4-thiadiazole to yield imidazolo[2,1-b]thiadiazole derivatives 4.The new heterocyclic compounds 4 are used as a key starting materials to synthesize some hetrocycles include pyrrolo-thiadiazolo imidazole , pyridazinone and spiro derivatives .The behavior of the pyridazinone compounds towards different electrophilic and nucleophilic reagents were investigated. The structure of the newly synthesized compounds were elucidated by elemental analysis and spectroscopic data.
Keywords: 4-Acetamido phenyl-4-oxo-but-2-enoic acid , oxirane,imidazol[2,1-b]thiadiazole, Pyrrolo imidazole,spiro pyrazolo and isoxazolo imidazo thiadiazole,pyridazinone
Cite this paper: Sameh. A. Rizk , "Utility of E-1-(4-Acetamidobenzoyl)-2-Oxirane Carboxylic Acid in Synthesis Some Fused Heterocycles and Spiro Compounds", American Journal of Chemistry, Vol. 1 No. 2, 2011, pp. 65-71. doi: 10.5923/j.chemistry.20110102.14.
1. Introduction
- (E)-4-aryl-4-oxo-2-butenoic acids have been shown that the substitution pattern on the aroyl moiety influences the antiproliferative activity[1]and they have activated double bond,Half-wave reduction potentials (E1/2)[2] display good correlations with Hammett sigma value, attempts to obtain good correlations using frontier orbitals of the molecules.Also,they have emerged the most promising drug candidates[3] which are selective for integrase S-1360[4] and class of Human immunodeficiency virus type1(HIV-1) integrase inhibitors[5] .Spiroindoline[6]and imidazoline[7] derivatives can be evaluated for their binding affinities and antagonistic activities at neuropeptide Y Y5 receptor and good brain penetration. Also, spironolactone is as effective as thiazides in treating mild hypertension without inducing hypokalemia or increased secretion of aldosterone[8,9] and eplerenone ,a specific aldosterone antagonist approved by the food and drug administration ,appears to have a much lower affinity for androgen and progesterone receptors , reduced incidence of sexual disturbances[10] and useful agent in treatment of hepertension and congestive heart failure , treatment of diabetics complication and aldose reductase inhibitors[13]. The most notably ketoconazole[11,12] which have been successful as antifungal agents and when spiroimidazolderivatives[14] was combined with an tibacterial agent (vancomycin, ciprofloxacin) that observed antagonistic activity results from the competitive binding of the medicine molecules into fungi cells receptors.3-phenylamino-(substituted phenyl)isoxazolines[15]were evaluated for their in vitro antifungal activity and on the proliferative response of human mononuclear peripheral blood cells to phytohemagglutinin A (PHA)[16].Recently,it reported[17] the synthesized new class of oxadiazoles by cyclization of the terminal carboxylic group of 3-aroyl propionic acids into oxadiazole nucleus that an objective of developing better anti-inflammatory and analgesic agents. Also, pyridazin-2-ylmethyl-2-substituted1,3,4-oxadiazole[18] screened for antibacterial, antifungal and antitubercular activity. The effects of 1,3,4 thiadiazole derivatives on the central nervous system (CNS) of mice were studied[19].Imidazolooxazole derivatives[20] via treatment of imidazolederivatives with oxirane have been tested for antimycobacterial activity .
2. Results and Discussion
- Reports from our laboratory[21-25] revealed that the β-aroyl acrylic acids are convenient poly electrophilic reagents in the synthesis of heterocycles, which for the addition reaction of nucleophililes e.g.carbon,nitrogen,sulfur occur exclusively at the α-carbon electrophilic center of the carboxy precursors.Morever , reaction with hydrogen peroxide afford oxirane derivative[26]. With the aim of broad ing the synthetic potential of β-aroyl acrylic acids ,the authors can be reported the behavior of 3-(4- acetylaminobenzoyl) prop -2- enoic acid 1 that was allowed to react hydrogen peroxide in the presence of sodium hydroxide afforded the epoxide product E-1-(4-acetylaminobenzoyl) 2-oxirane carboxylic acid 2. When the acid 2 is submitted to react with 5-aryl-2-amino-1,3,4-thiadiazole in the presence of few drops of piperidine afforded 2-(5-aryl-1,3,4- thiadiazol-2-yl)amino-3-hydroxy-3-(4-acetylaminobenzoyl) propanoic acid 3, via the N- alkylation of aminothiadiazole moieties that added to the activated 3-membered heterocycle of the acid 2. The acids 3 undergo spontaneous dehydration to afford imidazolo[2,1-b]thiadiazole derivatives 4 that more thermodynamically stable. (scheme-1)The different kinds of electrophilic centers in the compounds 4 can be reacted with simply binucleophiles e.g. hydrazine derivatives and hydroxyl amine to afford an important heterocycles and spiro compounds. The α -substituted hydrazone intermediates 5 undergo to internal ring closure via[3+2] instead of[4+2] cyclization process to generate pyrrole derivatives 7 rather than 4,5-dihydropyridazines 8[27,28]. The ring closure is promoted in the intermediates 5 by the presence of an acidic hydrogen originally placed in position 4 of the azo-ene system , and promote the thermo chemically allowed[4+2] cyclization to afford the competitive product 9 . Formation of the pyridazinone 9 is due to stability of the bond length and binding energy[29] than isomer 8. Moreover, the authors can be isolated uncommon spiro compounds 10 which have been afforded via the intermediate 6 as outlined in scheme 3.
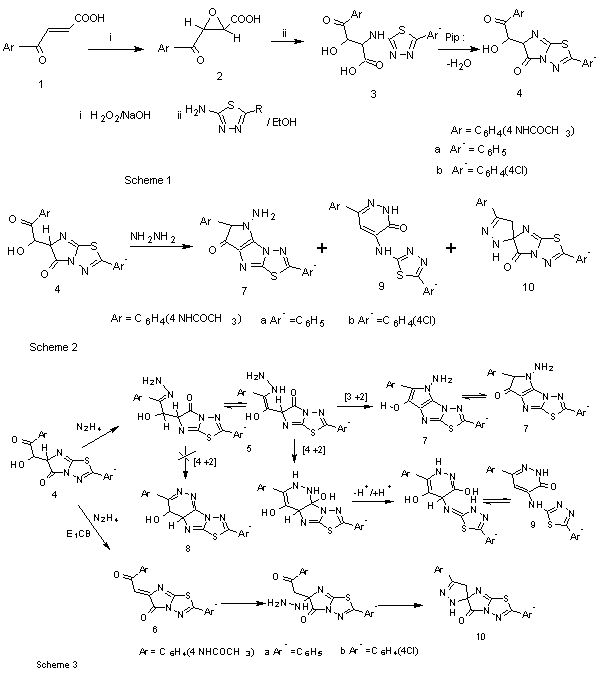
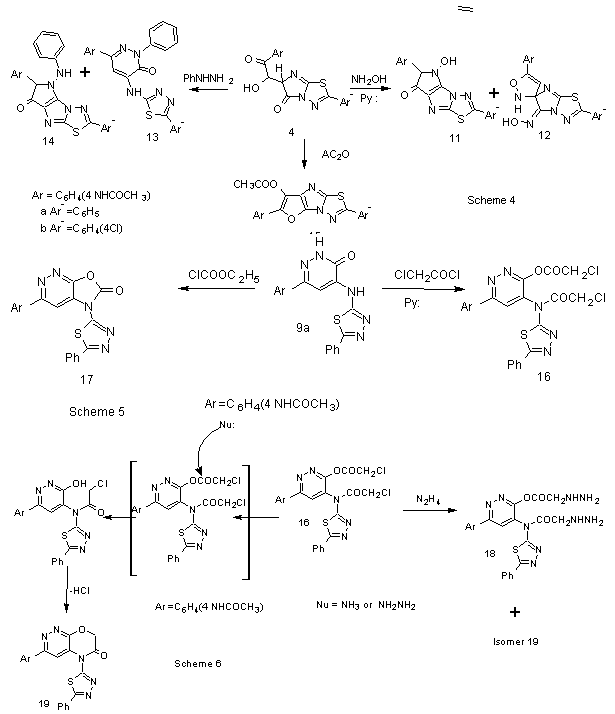 Similarity , the compounds 4 were allowed to react with hydroxyl amine in the presence of pyridine afford pyrrole derivatives 11 and spiro isomers 12 (scheme 4). Unsymmetrical hydrazine derivatives also can be affected on regio selectivity in which electronic and steric factors play an important rule .This can be affected on the reaction path that depends on stability of intermediate and the product. Thus, when the compounds 4 were allowed to react with phenyl hydrazine afforded the pyridazinone derivatives 13 and pyrrole derivatives 14. The latter compounds have low yield due to the steric phenyl group is outweigh intramolecular hydrogen bond and becomes a driving force to regioselective isomer 13. Also , the compounds 4 have been reacted with carbon electrophiles ,when 4 were allowed to react with acetic anhydride, they afforded furo[2,3-d] thiadiazolo[3,2-a]imidazole derivatives 15 scheme 4.Synthetic 3(2H) pyridazinones are important scaffolds in drug discovery, with many of their analogs being in the treatment of various human pathological states.They were described antihypertensive[30], antibacterial,antifungal[31], new azo ligant dye[32], cardiotonic and vasorelaxant activity[33], anti-tumor[34] and Selective cyclin dependent kinase inhibitor[35]. This prompted to continuo[21-24] the preparation of pyridazinone derivatives incorporated with 1,3,4-thiadiazole nucleus in position 4. Thus, when pyridazinone 9a was allowed to react with chloro acetyl chloride and ethyl chloro formate, afforded ester 16 and oxazolopyridazine 17 respectively (Scheme 5).When the chloro ester of pyridazine derivative 15 was allowed to react with hydrazine hydrate and ammonium acetate, it afforded hydrazine derivative 17 and1,4-oxazino[2,3-c]pyridazine 18 (Scheme 5).
Similarity , the compounds 4 were allowed to react with hydroxyl amine in the presence of pyridine afford pyrrole derivatives 11 and spiro isomers 12 (scheme 4). Unsymmetrical hydrazine derivatives also can be affected on regio selectivity in which electronic and steric factors play an important rule .This can be affected on the reaction path that depends on stability of intermediate and the product. Thus, when the compounds 4 were allowed to react with phenyl hydrazine afforded the pyridazinone derivatives 13 and pyrrole derivatives 14. The latter compounds have low yield due to the steric phenyl group is outweigh intramolecular hydrogen bond and becomes a driving force to regioselective isomer 13. Also , the compounds 4 have been reacted with carbon electrophiles ,when 4 were allowed to react with acetic anhydride, they afforded furo[2,3-d] thiadiazolo[3,2-a]imidazole derivatives 15 scheme 4.Synthetic 3(2H) pyridazinones are important scaffolds in drug discovery, with many of their analogs being in the treatment of various human pathological states.They were described antihypertensive[30], antibacterial,antifungal[31], new azo ligant dye[32], cardiotonic and vasorelaxant activity[33], anti-tumor[34] and Selective cyclin dependent kinase inhibitor[35]. This prompted to continuo[21-24] the preparation of pyridazinone derivatives incorporated with 1,3,4-thiadiazole nucleus in position 4. Thus, when pyridazinone 9a was allowed to react with chloro acetyl chloride and ethyl chloro formate, afforded ester 16 and oxazolopyridazine 17 respectively (Scheme 5).When the chloro ester of pyridazine derivative 15 was allowed to react with hydrazine hydrate and ammonium acetate, it afforded hydrazine derivative 17 and1,4-oxazino[2,3-c]pyridazine 18 (Scheme 5).3. Conclusions
- From the spectroscopic tools, the reaction of isomer 19 possibly takes place via attacking nucleophiles by tetrahedral mechanism followed by ring closure yielded the corresponding 1,4-oxazino[2,3-c]pyridazine 19 (Scheme 6). The present work is succeeded to synthesis of a series of some important heterocycles and spiro compounds from 4-acetamido phenyl-4-oxo-2-butenoic acid and for the first time,synthesis of pyridazinone derivatives bearing 4-heteryl moiety inside to aromatic substituents in the position 6 that enhances the biological profile many fold than their parent nuclei.
4. Experimental
- All melting points are uncorrected.and were determined on a stuart electric melting point apparatus.Elemental analyses were carried out at the Microanalytical Center, National Research Center, Cairo, Egypt. By Elementar Viro El Microanalysis IR spectra (KBr) were recorded on infrared spectrometer FT-IR 400D using OMNIC program and are reported frequency of absorption in terms of cm-1 and H-NMR spectra recorded on a Bruker spectrophotometer at 400 MHz using TMS as internal standard and withresidual signals of the deuterated solvent δ = 7.26 ppm for CDCl3 and δ 2.51 ppm for DMSO-d6. C-NMR spectra were recorded on the same spectrometer at 100 MHz and referenced to solvent signals δ = 77 ppm for CDCl3 and δ 39.50 ppm for DMSO-d6.DEPT 135 NMR spectroscopy were used where appropriate to aid the assignment of signals in the H and C-NMR spectra. The mass spectra were recorded on Shimadzu GCMS-QP-1000 EX mass spectrometer at 70 e.v using the electron ionization technique. Homogeneity of all compounds synthesized was checked by TLC.General Procedure of starting Material in literature[21].E-1-(4-Acetamidobenzoyl)-2-Oxirane Carboxylic Acid (2)A solution of 3-(4-acetamidobenzoyl)-prop-2-enoic acid (2.35 g;0.01 mol) in acetone(40 mL) and methyl alcohol (15 mL) was treated with 8% aqueous sodium hydroxide (12 mL) followed by hydrogen peroxide (30%,5 mL). The reaction mixture was allowed to boil 1 h and then left over night at room temperature. The crude product was washed by petroleum ether (b.p 40- 60oC), and then, crystallized from toluene to give compound 2Yield 83%.Mp 172-174 C. IR(KBr) 1645,1687,1710(CO). 1HNMR spectrum (CDCl3): δ 2.51(s,3H,CH3), 6.91-6.99 (2dd, 1Ha and 1Hb diastereotopic protons, J=14.5 and 9.2) , multiplet at 7.30 – 7.70 assigned for 4ArH aromatic protons, singlet 8.2 a acidic proton which exchanged in D2O and.Anal.Calc. for C12H11NO5 :C57.38 , H 4.41 ;found: C57.22,H 4.21. MS: m/z 249[M], 205[M- CO2], 163[205 -COCH3]Compounds 4An equimolar mixture of compound 2 (2.5 g; 0.01 mol) and 2-amino-5-aryl-1,3,4-thiadiazole (0.01 mol) in 50 mL ethanol . The reaction mixture was refluxed for 3 h. The solid that separated after cool was filtered off,washed by petroleum ether (b.p 40- 60oC),dried and then, crystallized from ethanol afford 45-[2-(4-acetylaminophenyl)-2-oxo-1-hydroxy]ethyl-4-oxo-2-phenyl imidazolo[2,1-b]1,3,4-thiadiazole (4a)Yield 74%.Mp 190-192 C. IR(KBr) 1613 (C=N), 1650, 1670, 1685 (CO), 3245 (NH), 3410 (OH). 1HNMR (DMSO): δ 2.5(s,3H,CH3), 4.11(dd,1Ha, (J=15.2, J=11.2) and 1Hb (J=15.2, J=11.1) sterogenic methine protons), 4.35 (bs,1H, OH proton of hydroxyl group), multiplet at 7.44 – 7.73 assigned for 9ArH aromatic protons, singlet 13.2 a acidic OH=NH proton which exchanged in D2O and Anal. Calc. for C20H16N4SO4: C 58.82, H 3.92, N 13.72; found: C 58.60, H 3.65, N 13.45. MS:m/z 408[M], 377[M-OH+CH3], 285, 213, 141.5-[2-(4-acetylaminophenyl)-2-oxo-1-hydroxy]ethyl-4-oxo-4-chlorophenylimidazolo[2,1-b]1,3,4thiadiazole (4b)Yield 74%.Mp 200-202 C. IR(KBr) 1620 (C=N), 1645, 1675, 1691(CO), 3245 (NH). 1HNMR (DMSO): δ 2.06(s,3H, CH3), 3.50 (bs,1H,OH proton of hydroxyl group), 6.6-6.7 (2dd,1Ha,(J=15.2,J=11.2) and 1Hb (J=15.2, J=11.1) sterogenic methine protons), multiplet at 7.44 – 7.83 assigned for 8ArH aromatic protons, singlet 8.2 a acidic OH=NH proton which exchanged in D2O and Anal. Calc. for C20H15N4SClO4: C 54.30, H 3.39, N 12.67; found: C 54.55, H 3.25, N 12.53. MS:m/z 442[M],426[M-OH], 365, 287, 252, 170, 159, 139.Compounds 7,9,10A mixture of 4(0.01 mol) and hydrazine hydrate (0.01mol) in ethanol (50 mL) and was heated under reflux for 5h. The reaction mixture was allowed to cool and the product was filtered, dried, and were recrystallized from suitable solvent, using the column chromatograph is necessary to separate the compounds 7 and 10. 2-phenyl-4-amino-5-(4-acetylaminophenyl)-6-oxo- pyrrolo[3,2-d]-1,3,4-thiadiazolo[3,2-a] imidazole (7a)Yield 35%.Mp 150-152 C. IR(KBr) 1650,1670 (CO), 3275,3200 (NH),3400 (OH). Anal.Calc. for C20H16N6SO2 : C 59.40 , H 3.96,N 20.79;found: C 59.25 , H 3.75, N 20.50 2-(4-chlorophenyl)-4-amino-5-(4-acetylaminophenyl)-6-oxo- pyrrolo[3,2-d]-1,3,4-thiadiazolo[3,2-a]imidazole (7b)Yield 35%.Mp 168-170 C. IR(KBr) 1650,1670 (CO), 3275,3200 (NH),3400 (OH). and Anal.Calc. for C20H15N6ClSO2 : C 54.79 , H 3.42,N 19.17;found: C 54.47 , H 3.21, N 19.03 .Compounds 96-(4-acetylaminophenyl)-4-(5-phenyl-1,3,4-thiadiazol-2-yl)amino-2,3-dihydropyridazin-3(2H)one (9a)Yield 30%. Mp 203-205 C. IR(KBr) 1640,1687(CO) ,3272 (NH). and Anal.Calc. for C20H16N6SO2 : C 59.40 , H 3.96,N 20.79;found: C 59.25 , H 3.75, N 20.60 MS:m/z 404[M],360[M-COCH3].6-(4-acetylaminophenyl)-4-(5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)amino-2,3-dihydropyridazin-3(2H)one (9b)Yield 30%. Mp 220-222 C. IR(KBr) 1627,1687(CO) ,3266 (NH). 1HNMR (DMSO-d6): δ 2.51(s,3H,CH3), 6.6 (bs,3H,NH of amino, acetamido and pyridazinone moiety) 7.26-8.13 (m,9H,Ar-H and pyridazine). Anal.Calc. for C20H15N6ClSO2: C 54.79 , H 3.42,N 19.17;found: C 54.52 , H 3.22, N 19.00 MS:m/z 438[M],395[M-COCH3].Compounds 104-phenyl-7-oxo-10-(4-acetylaminophenyl)-spiro[7(2-6)-4]3-thia-1,5,6,8,9pentazadodecane(10a)Yield 25%. Mp 171-175 C. IR(KBr) 1640,1671(CO) ,3423 (NH). 1HNMR (DMSO-d6) : δ,2.2(s,2H,CH2)2.5(s,3H,CH3) 6.2 (bs,1H,NH) 7.0-7.81 (m,9H,Ar-H), 10.40(brs,1H,NH of acetamido moiety)and Anal.Calc. for C20H16N6SO2: C 59.40 , H 3.96,N 20.79;found: C 59.25 , H 3.75, N 20.60 MS:m/z 307[M- (aniline radical+H2)],121[PhC=N-NH].4-(4-chlorophenyl-7-oxo-10-(4-acetylaminophenyl)-spiro[7(2-6)-4]3-thia-1,5,6,8,9pentazadodecane(10b)Yield 25%.Mp 165-168 C. IR(KBr) 1640,1671(CO) ,3420 (NH). Anal.Calc. for C20H15N6ClSO2 : C 54.79 , H 3.42,N 19.17;found: C 54.52 , H 3.22, N 19.00 MS:m/z 404[M-Cl],395[M-COCH3],194[spiro moiety].Compounds of 11and 12A mixture of 4(0.01 mol) and hydroxyl amine hydrochloride (1.03 g ;0.015mol) in boiling pyridine (50 mL )and was heated under reflux for 6h. The reaction mixture was allowed to cool, pour into ice/HCl and the product was filtered, dried , and were recrystallized from toluene afford 11 and ethanol afford 12 2-phenyl-4-hydroxy-5-(4-acetylaminophenyl)-6-oxo- pyrrolo[3,2-d]-1,3,4-thiadiazolo[3,2-a] imidazole (11a)Yield 40%.Mp 165-167 C. IR(KBr) 1650,1683 (CO), 3245 (NH),3450 (OH). and Anal.Calc. for C20H15N5SO3 : C 59.25 , H 3.70,N 17.28;found: C 59.00 , H 3.45, N 17.00 MS:m/z 403[M-H2],362[M-COCH3],268[361-phenol moiety].2-(4-chlorophenyl)-4-hydroxy-5-(4-acetylaminophenyl)-6-oxo- pyrrolo[3,2-d]-1,3,4-thiadiazolo[3,2-a]imidazole (11b)Yield 45%.Mp 168-170 C. IR(KBr) 1650,1670 (CO), 3275(NH),3450 (OH). and Anal.Calc. for C20H14N5ClSO3 : C 54.66, H 3.19,N 15.94;found: C 54.36 , H 3.02, N 15.68 4-phenyl-7-hydroxyimino-10-(4-acetylaminophenyl)-spiro[7(2-6)-4]9-oxa-3-thia-1,5,6,8, tetrazadodecane(12a)Yield 35 %.Mp 197-200 C. IR(KBr) 1630(CO) ,3425 (NH). 1HNMR (DMSO-d6) : δ 2.23(s,3H,CH3), 4.29 (bs,2H,OH and NH groups) 7.00-7.70 (m,10H,Ar-H), 12.70 (brs,1H,NH of acetamido moiety) and Anal.Calc. for C20H16N6SO3 : C 57.14 , H 3.80,N 20.00;found: C 57.00 , H 3.55, N 19.72 .4-(4-chlorophenyl-7-hydroxyimino-10-(4-acetylaminophenyl)-spiro[7(2-6)-4]9-oxa-3-thia-1,5,6,8, tetrazadodecane(12b)Yield 35%.Mp 192-195 C. IR(KBr) 1631 (CO) ,3271 (NH). Anal.Calc. for C20H15N6ClSO3 : C 52.86 , H 3.30,N 18.50;found: C 54.52 , H 3.16, N 18.25 MS:m/z454[M],343[M-chlorobenzene].Compounds 13:A mixture of 4(0.01 mol) and phenyl hydrazine (0.01mol) in ethanol (40 mL )and was heated under reflux for 5h. The reaction mixture was allowed to cool and the separated product was filtered, dried and were recrystallized from benzene/toluene afford 14 and ethanol afford 13. 2-phenyl-4-(5-phenyl-1,3,4-thiadiazol-2-yl)amino-6-(4-acetylaminophenyl)-2,3-dihydropyridazin-3(2H)one (13a)Yield 40%. Mp 212-214 C. IR(KBr) 1640,1687(CO) ,3170 (NH). and Anal.Calc. for C26H20N6SO2 : C 65.00 , H 4.16,N 17.50;found: C 64.70 , H 4.00, N 17.36 .2-phenyl-4-(5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)amino-6-(4-acetylaminophenyl)-2,3-dihydro-pyridazin-3(2H)one (13b)Yield 45%. Mp 230-232 C. IR(KBr) 1632,1721(CO) ,3220 (NH). Anal.Calc. for C26H19N6ClSO2 : C 60.70 , H 3.69,N 16.34;found: C 60.44 , H 3.32, N 16.10 .2-phenyl-4-phenylamino-5-(4-acetylaminophenyl)-6-oxo-pyrrolo[3,2-d]-1,3,4-thiadiazolo-[3,2-a] imidazole (14a)Yield 35%.Mp 170-172 C. IR(KBr) 1650,1670 (CO), 3275,3200 (NH),3400 (OH). and Anal.Calc. forC26H20N6SO2 : C 65.00 , H 4.16,N 17.50;found: C 65.00 , H 4.10, N 17.26 2-(4-chlorophenyl)-4-phenylamino-5-(4-acetylaminophenyl)-6-oxo- pyrrolo[3,2-d]-1,3,4-thiadiazolo[3,2-a]imidazole (14b)Yield 35%.Mp 190-191 C. IR(KBr) 1650,1670 (CO), 3275,3200 (NH),3400 (OH). and Anal.Calc. forC26H19N6ClSO2 C 60.70, H 3.69,N 16.34;found: C 60.44 , H 3.32, N 16.10 Compounds 15a-bA mixture of 4(0.01 mol) and acetic anhydride (9.4 mL,0.1mol) and then refluxed on water bath for 2h. The excess acetic anhydride was removed by distillation and the separated product was filtered,dried and were recrystallized from mix toluene-ethanol 2-acetoxy-3-(4-acetylaminophenyl)-6-phenyl-furo[3,2-d]-1,3,4-thiadiazolo[3,2-a] imidazole (15a)Yield 74%.Mp 150-152 C. IR(KBr) 1613 (C=N), 1650, 1764, 1850 (CO), 3154 (NH),3436 (OH). 1HNMR (DMSO): δ 2.22(s,3H,CH3), 2.50 (s,3H,CH3COO), multiplet at 7.50 – 7.90 assigned for 9ArH aromatic protons, singlet 12.67 a acidic OH=NH proton which exchanged in D2O and Anal.Calc. for C22H16N4SO4: C 61.11 , H 3.70,N 12.96;found: C 60.90 , H 3.47, N 12.77. 2-acetoxy-3-(4-acetylaminophenyl)-6-(4-chlorophenyl)-furo[3,2-d]-1,3,4-thiadiazolo[3,2-a]imidazole(15b)Yield 74%. Mp 144-146 C. IR(KBr) 1620(C=N),1645,1767,1850 (CO), 3157 (NH) 3438 (OH). 1HNMR (DMSO): δ 2.21(s,6H,CH3CONH CH3COO), multiplet at 7.51 – 7.94 assigned for 8ArH aromatic protons, singlet 12.57 a acidic OH=NH proton which exchanged in D2O and Anal.Calc. for C22H15N4SClO4 : C 56.65 , H 3.21,N 12.00;found: C 56.40 , H 3.00, N 11.77. 3-chloroacetoxy6-(4-acetylaminophenyl)-4-(5-phenyl-1,3,4-thiadiazol-2-yl)chloroacetylaminopyridazine (16)An equimolar mixture of compound 9a (2.0 g;5mmol) and chloroacetylchloride (1.7mL,0.015 mol) in 50 mL dry pyridine . The reaction mixture was refluxed for 3 h. The reaction mixture poured into ice/HCl and the solid that separated was filtered off, dried and then, crystallized from ethanol .Yield 45%. Mp 132-134 C. IR(KBr) 1660,1722,1781 (CO) ,3317 (NH). and Anal.Calc. for C24H18N6SCl2O4 : C 51.80 , H 3.23,N 15.10;found: C 51.84 , H 3.13, N 15.40 MS:m/z 485[M-2Cl],380[M-OCOCH2CO moiety],360[M-(CH3CO+2ClCH2CO)].2-oxo-3-(5-phenyl-1,3,4-thiadiazol-2-yl) -5-(4-acetylaminophenyl)-)oxazolo[5,4-c]pyridazine (17)An equimolar mixture of compound 9a (2.0 g;5mmol) and ethylchloroformate (1.4 mL,0.015 mol) in 50 mL dry pyridine . The reaction mixture was refluxed for 3 h. The reaction mixture poured into ice/HCl and the solid that separated was filtered off, dried and then, crystallized from ethanol .Yield 75%. Mp 142-144 C. IR(KBr) 1660,1725(CO) ,3160 (NH). and Anal.Calc. for C21H14N6SO3 : C 58.60 , H 3.25,N 19.53;found: C 58.38 , H 3.17, N 19.30 MS:m/z 430[M]. 2-oxo-4-(5-phenyl-1,3,4-thiadiazol-2-yl)-6-(4-acetylaminophenyl)-)1,2,3,4tetrahydro1,4-oxazino[2,3-c] pyridazine (18)Yield 35%. Mp 190-192 C. IR(KBr) 1650,1723 (CO), 3320,3188 (NH2,NH). 1HNMR (DMSO-d6) : δ 2.08(s,3H, CH3), 4.13-4.17(s,4H,2CH2-N) 6.72-6.76(m,6H, 2NHNH2), 7.46-7.92 (m,10H,Ar-H and pyridazine),11.36 (brs,1H,NH of acetamido moiety) and Anal.Calc. for C24H24N10SO4 : C 52.55 , H 4.37,N 25.54;found: C 52.30 , H 4.16, N 25.00 3-oxo-4-(5-phenyl-1,3,4-thiadiazol-2-yl)-6-(4-acetylaminophenyl)-)1,2,3,4tetrahydro1,4-oxazino[2,3-c] pyridazine (19)An equimolar mixture of compound 16 (2.75 g;5mmol) and hydrazine hydrate (1.7mL,0.015 mol) and /or ammonium acetate (1.2 g ;0.015 mol ) in 50 mL dry pyridine . The reaction mixture was refluxed for 3 h. The reaction mixture poured into ice/HCl and the solid that separated was filtered off, dried and then, crystallized from ethanol .Yield 67 %.Mp 168-170 C. IR(KBr) 1644,1708,1729 (CO) ,3177 (NH). 1HNMR (DMSO-d6) : δ 2.2(s,3H,CH3 ), 4.2 (s,2H,CH2-N),7.43-7.81 (m,10H,Ar-H and pyridazine),11.59 (brs,1H,NH of acetamido moiety) and Anal.Calc. for C22H16N6SO3 : C 59.45 , H 3.60,N 18.91;found: C 59.28 , H 3.43, N 18.72 MS:m/z 444[M].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML