-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(2): 60-64
doi: 10.5923/j.chemistry.20110102.13
Complex Bilding Behavior of L-Tryptophan and Related Amino Acids, a Comparative Investigation
S. A. A. Sajadi
Sharif University of Technology, Institute of Water & Energy, Tehran P. O. Box 11155-8639, Iran
Correspondence to: S. A. A. Sajadi , Sharif University of Technology, Institute of Water & Energy, Tehran P. O. Box 11155-8639, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
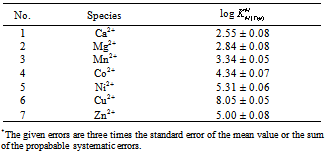
The acidity constants of Tryptophan1 were determined by potentiometric pH titration. The stability constants of the 1:1 complexes formed between M2+: Ca2+, Mg2+, Mn2+, Co2+, Ni2+, Cu2+ or Zn2+ and Trp2−, were determined by potentiometric pH titration in aqueous solution (I = 0.1 M, NaNO3, 25°C). The order of the stability constants was reported. It is shown that the stability of the binary M(Trp) complexes is solely determined by the basicity of the carboxyl or amino group. All the stability constants reported in this work show the usual trend. The obtained order is Ca2+< Mg2+
Keywords: Tryptophan, Amino Acids, Divalent Metal Ions, Potentiometric Titration, Acidity and Stability Constants
Cite this paper: S. A. A. Sajadi , "Complex Bilding Behavior of L-Tryptophan and Related Amino Acids, a Comparative Investigation", American Journal of Chemistry, Vol. 1 No. 2, 2011, pp. 60-64. doi: 10.5923/j.chemistry.20110102.13.
1. Introduction
- Tryptophan is one of the 10 essential amino acids that the body uses to synthesize the proteins it needs (fig. 1)[1]. It's well-known for its role in the production of nervous system messengers, especially those related to relaxation, restfulness, and sleep. Tryptophan has two important functions. First, a small amount of the tryptophan we get in our diet (about 3%) is converted into niacin (vitamin B3) by the liver. This conversion can help prevent the symptoms associated with niacin deficiency when dietary intake of this vitamin is low. Second, tryptophan serves as a precursor for serotonin, a neurotransmitter that helps the body regulate appetite, sleep patterns, and mood. Because of its ability to raise serotonin levels, tryptophan has been used therapeutically in the treatment of a variety of conditions, most notably insomnia, depression, and anxiety. Vitamin B6, vitamin C, folic acid and magnesium are necessary for the metabolization of tryptophan. In addition, tyrosine and phenylalanine compete with tryptophan for absorption[2,3].Because of the essential rols of tryptophan in biological systems is important to investigate its interactions with different metal ions and the regarding complex bilding.It is a vital constituent of proteins and indispensable in human nutrition for establishing and maintaining a positive nitrogen balance[4]. Besides, some of its derivatives are potent drugs[5]. Trp is widely used in food industry. It is sometimes added to dietary and feed products as a food fortifier in order to maintain the amino acid balance of the food and correct possible dietary deficiencies. Trp can also be used to study structure and dynamics of the proteins because of its indole moiety[6]. In particular, Trp is the precursor of the neurotransmitter serotonin and plays an important role in brain function and related regulatory mechanisms[7]. In addition, Trp is an important and frequently used starting material in the chemical synthesis of a range of pharmaceuticals.Among the side chains of amino acids, the indole moiety is the most potent electron donor[8]. Indeed, charge- transfer-type interactions between tryptophan or other indole derivatives and nucleosides or nucleotides occur in aqueous solution[9-15].
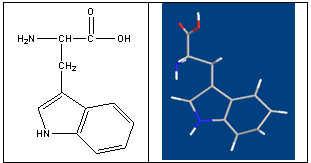 | Figure 1. Chemical structure of and L-Tryptophan |
2. Experimental
- MaterialsThe L- tryptophan (extra pure) was purchased from Merck, Darmstadt, Germany. The nitrate salt of Na+, Ca2+, Mg2+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+ (all pro analysis) were from Merck. All the starting materials were of pro analysis grade and used without further purification. Potassium hydrogen phthalate and standard solutions of sodium hydroxide (titrasol), nitric acid, EDTA and of the buffer solutions of pH 4.0, 7.0 and 9.0 were all from Merck. All solutions were prepared with de-ionized water. Water was purified by Milil-Q water purification system, de-ionized and distillated.pH titrationsReagentsCarbonate-free sodium hydroxide 0.03 M was prepared and standardized against sodium hydrogen phthalate and a standard solution of nitric acid 0.5 mM. M(II) nitrate solution (0.03 M) was prepared by dissolving the above substance in water and was standardized with standard solution of EDTA 0.1 M (triplex).ApparatusAll pH titrations were performed using a Metrohm 794 basic automatic titrator (Titrino), coupled with a thermo-stating bath Hero at 25C (±0.1C) and a Metrohm combined glass electrode (Ag/AgCl). The pH meter was calibrated with Merck standard buffer solutions (4.0, 7.0 and 9.0). ProcedureFor the determination of acid dissociation constants of the ligand Trp, an aqueous solution (0.3 mM) of the protonated ligand was titrated with 0.03 M NaOH at 25C under nitrogen atmosphere and ionic strength of 0.1 M, NaNO3. For the determination of binary (a ligand and M2+) system, the ratios used were 1:1, M(II) : Trp, 0.3 mM. This solution was titrated with 0.03 M NaOH under the same conditions mentioned above. Each titration was repeated seven times in order to check the reproducibility of the data. CalculationThe acid dissociation constants,
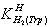 and
and 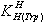 for H2(Trp) were calculated by an algebraic method. The equilibrium involved in the formation of 1:1 complex of Trp and a divalent metal ion may be expressed as equations (1) & (2).
for H2(Trp) were calculated by an algebraic method. The equilibrium involved in the formation of 1:1 complex of Trp and a divalent metal ion may be expressed as equations (1) & (2).3. Results and Discussion
- The potentiometric pH-titrations (25C, 0.1 M, NaNO3) were carried out to obtain the acidity and stability constants which are summarized in table 1 & 2.
|
|
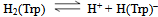 | (1a) |
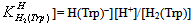 | (1b) |
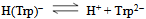 | (2a) |
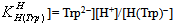 | (2b) |
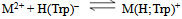 | (3a) |
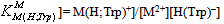 | (3b) |
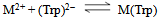 | (4a) |
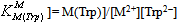 | (4b) |
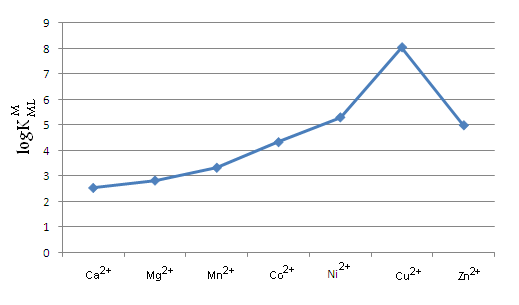 | Figure 2. Irving-Williams sequence-type plot for the 1:1 complexes of Ca2+ to Zn2+ with Tryptophan (see table 2) |
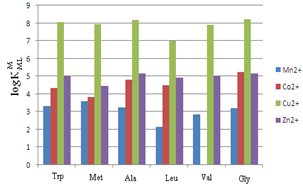 | Figure 3. stability constants of the 1:1 complexes of Mn2+ to Zn2+ with some amino acids (see table 3). Leu: Leucine, Val: Valine, Ala: Alanine, Gly: Glycine, Met: Methionine, Trp: Tryptophan |
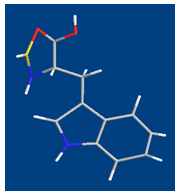 | Figure 4. Schematic structures of the species with interactions according to equilibrium (4) for Cu(Trp). The structure was drawn with the program CS Chem 3D, version 3.5, from Cambridge Software Corporation |
4. Conclusions
- The Tryptophan industry continues to deny that exposure to free Tryptophan found in processed food causes adverse reactions including hives, asthma etc., which is interesting to investigate. Another interesting point is the pharmacological application of new generation of Tryptophan complexes and it seems essential to understand their reaction mechanism.
References
| [1] | B. Widner, B. Wirleitner, G. Baier-Bitterlich, et al. Arch Immunol Ther Exp (Warsz) ;48(4), (2000), 251-8 |
| [2] | J.L. Celenza, Curr. Opin. Plant Biol., 4(3), (2001), 34-40 |
| [3] | IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Nomenclature and Symbolism for Amino Acids and Peptides. Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007-05-17 |
| [4] | A.R. Fiorucci, E.T.G. Cavalheiro, J. Pharm. Biomed. Anal. 28, (2002) 909–915 |
| [5] | H.H. Hussey, J. Am. Chem., Soc., 87, (1974) 1126 |
| [6] | P. Cioni, G.B. Strambini, Biochim. Biophys. Acta, 1595, (2002), 116–130 |
| [7] | R. Sapolsky, "Biology and Human Behavior: The Neurological Origins of Individuality, 2nd edition". The Teaching Company. ", (2005), see pages 13 & 14 |
| [8] | R. Foster, Charge Transfer Complexes, Academic Press, 1969 |
| [9] | F. Morita, Biophys. Acta, 343, (1974), 674 |
| [10] | Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6 |
| [11] | L. Stryer, Biochemistry, 4th Edition, W.H. Freeman and Company New York, (1995) |
| [12] | H. Manev, E. Costa, Mol. Pharmacol., 36(1) (1989) 106-112 |
| [13] | P.J. Reeds et al., J. Nutrition, 130(45) (2000) 978S-982S |
| [14] | http://www.anyvitamins.com/millenium2000.htm |
| [15] | http://www.evitamins.com/healthnotes.asp?ContentID=1025008 |
| [16] | A.E. Martel, Critical Stability Constants of Metal Complexes 26 (2006) |
| [17] | S.A.A. Sajadi, A.A. Alamolhoda, A. Nazari Alavi, Scientica Iranica, (2010) in press |
| [18] | Handbook of Chem. & Physics, 55, (1975), D-129 |
| [19] | J. L. Miranda, J. Felcman, Polyhedron, 22, (2003), 225-233 |
| [20] | J. Felcman, J.L. Miranda, J. Braz., Chem. Soc., 8, (1997), 575 |
| [21] | IUPAC Stability Conatants Database, Release 3, version 3.02, coplied by L.D. Pettit, H.K. J. Powel, Academic Software Timble, UK, (1998) |
| [22] | H. Sigel, A.D. Zuberbuehler, O. Yamauchi, Anal. Chim. Acta, 255, (1991), 63 |
| [23] | H. Irving, R.J.P. Williams, J. Chem. Soc., (1953) 3192-3210 |
| [24] | A. Pecsvaradi et al., Proc. 7th Hungarian Congress, Plant Physiol., Acta Biol. Szegediensis, 46(3-4), (2002), 103-104 |
| [25] | E. Kvamme, G. Svenneby, I.A. Torgner, Neurochem. Res., 8(1), (1983), 25 |
| [26] | A. Ain, Med. J. Therap. Africa, 1(2), (2007), 162 |
| [27] | H. Sigel, C.F. Naumann, J. A. Chem. Soc., 98(3), (1976), 730-739 |
| [28] | F. Takeuchi, H. Otsuka, Y. Shibata, Acta Vitaminol. Enzymol., 3(4), (1981), 224-30 |
| [29] | M. Bisaillon, I. Bougie, J. Biol. Chem., 24, (1985), 499 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML