-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(2): 32-36
doi: 10.5923/j.chemistry.20110102.07
Promazine Complexes of Transition Metal Ions: Synthesis
Dayakar R. Gouru 1, Vishnuvardhan R. Thakkalapally 1, Tarab J. Ahmad 1, S. Ananda 2, Netkal M. Made Gowda 1
1Department of Chemistry, Western Illinois University, One University Circle, Macomb, 61455, USA
2Department of Studies in Chemistry, University of Mysore, Manasagangothri, Mysore, 570006, India
Correspondence to: Netkal M. Made Gowda , Department of Chemistry, Western Illinois University, One University Circle, Macomb, 61455, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Psychotherapeutic, antiemetic, and antihistamine activities are exhibited by some of the N- alkylaminophenothiazine derivatives including promazine (P). The N-alkylamine side chain is implicated in the aforementioned pharmacological activities. Several promazine (P.HCl or C17H20N2S.HCl) complexes of the transition metal ions, Zn(II), Cd(II) and Hg(II), have been synthesized. The complexes have been characterized by their elemental analysis, molar conductivity, magnetic susceptibility, UV-Visible, IR and 1H-NMR data. The molecular formulations of the new mononuclear complexes have been proposed. These complexes behave in DMF solutions as 1:1 electrolytes. Molecular structures have been proposed showing a square pyramidal environment around each metal center with an sp3d hybridization for the five-coordinate complexes, [ZnBr(C17H20N2S.HCl)2]Br, [CdBr(C17H20N2S.HCl)2]Br, [CdI(C17H20N2S.HCl)2]I.H2O and [HgBr((C17H20N2S. HCl)2]Br.
Keywords: Keywords Promazine, Metal Complexes, Synthesis, Characterization, Elemental and Spectroscopic Analyses
Cite this paper: Dayakar R. Gouru , Vishnuvardhan R. Thakkalapally , Tarab J. Ahmad , S. Ananda , Netkal M. Made Gowda , "Promazine Complexes of Transition Metal Ions: Synthesis", American Journal of Chemistry, Vol. 1 No. 2, 2011, pp. 32-36. doi: 10.5923/j.chemistry.20110102.07.
Article Outline
1. Introduction
- N-Alkylphenothiazines (NAPTZs) are biologically active heterocyclic compounds with the general structure shown in Fig.1. Their research was initially stimulated by the discovery of the anthelmintic action of N-substituted and C-substituted derivatives1. However, in recent years coordinating behavior of NAPTZs has gained much importance due to their extensive applications in industry, medicine, and chemical analysis2. Some of the NAPTZ ligands including promazine (P) are used as psychotherapeutic, antiemetic, and antihistamine drugs. The N-alkylamine side chain is considered to be responsible for the aforesaid pharmacological activities[1-3].
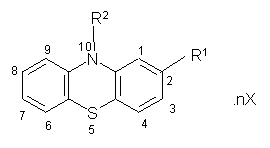 | Figure 1. General molecular structure of phenothiazine derivatives |
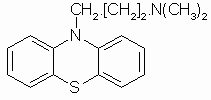 | Figure 2. Molecular structure of promazine |
2. Experimental
2.1. Materials
- Metal salts, zinc bromide, cadmium bromide, cadmium iodide and mercuric bromide, and the ligand, promazine hydrochloride (P·HCl; 99% purity) were obtained from Aldrich/Sigma Chemical Company, USA. All organic solvents such as methanol, ethanol, diethyl ether, dimethyl sulfoxide, dimethyl formamide and DMSO-d6 (Cambridge isotope laboratories Inc.) were of ACS reagent grade and were used without further purification. Double distilled water was used in all preparations.
2.2. Physical Measurements
- Elemental analyses of complexes were performed by Microanalysis Laboratory, University of Illinois, Urbana- Champaign, IL. Molar conductance was determined with the Conductance-Resistance meter. Melting points were determined on a Melt-Temp apparatus from Laboratory Devices, Cambridge, MA. Shimadzu UV1601 spectrophotometer was used to measure uv-visible spectra and absorbances of analyte solutions. The infrared spectra were recorded using potassium bromide discs on a Shimadzu FTIR 8400 spectrometer. 1H-NMR spectra were recorded on a JEOL-300 MHz FT-NMR spectrometer in DMSO-d6. Mass magnetic susceptibilities of the complexes were measured at room temperature with a Johnson Matthey magnetic susceptibility balance, which uses HgCo(SCN)4 as a calibrant.
2.3. General Synthesis of Complexes
- A known concentration of the transition metal salt (x mmol) (ZnBr2, CdBr2, CdI2 and HgBr2) in a minimum volume of MeOH was slowly added with stirring to a concentrated methanolic solution of P.HCl (2x mmol) and refluxed overnight. Each reaction mixture cooled overnight at 0℃ precipitated a product, which was isolated by suction filtration through a medium-glass fritted funnel. The product was washed with small amounts of cold water first followed by MeOH, air-dried, and dried in vacuo over anhydrous CaSO4 in a desiccator. Each crude product was recrystallized twice from a hot saturated solution in MeOH and dried as before. The yield was determined. The following general equation represents the stoichi- ometric reaction involved in the formation of complexes:
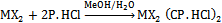 | (1) |
3. Results and Discussion
- The molecular formulations and structures of the complexes were determined on the basis of elemental analysis, molar conductance, UV-Vis, IR, and NMR data. The complexes are slightly soluble in common polar solvents such as MeOH and EtOH and readily soluble in acetone, acetonitrile, nitromethane, DMF, and DMSO (0.4 g – 2.0 g per 100 mL). All products except Zn(II) complex are insoluble in water. The physical properties of the new metal-P.HCl complexes are presented in Table 1. Complexes are colored, crystalline/microcrystalline, and relatively stable above room temperature as indicated by their melting point ranges with percent yields ranging from 82 to 93.
|
|
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||
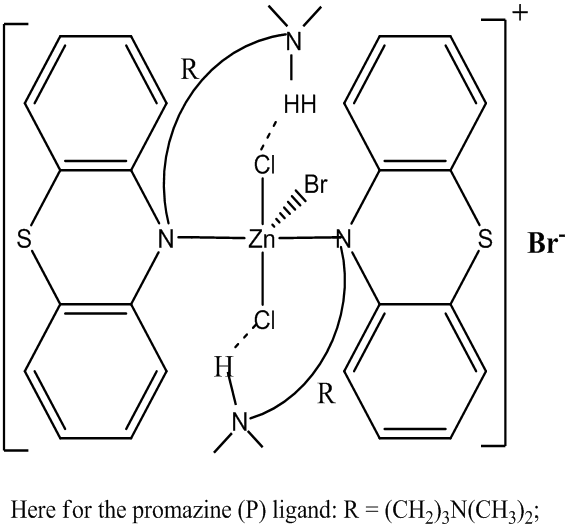 | Figure 3A. Proposed structure of [ZnBr(P.HCl)2]Br. R=(CH2)3N(CH3)2 |
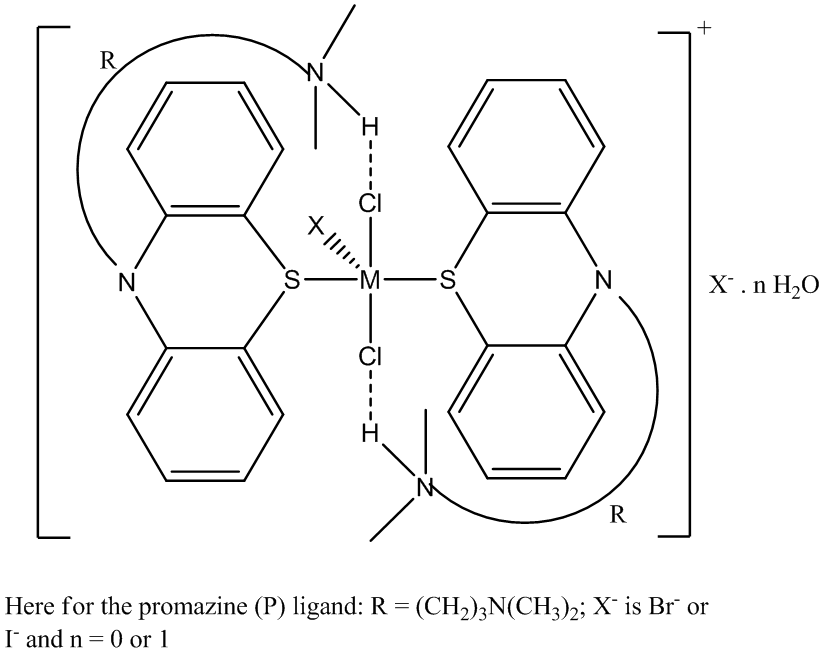 | Figure 3B. Proposed general molecular structure for the Complexes, [CdBr(P.HCl)2]Br, [CdI(P.HCl)2]I.H2O and [HgBr(P.HCl)2]Br |
4. Conclusions
- Transition metal-promazine hydrochloride complexes have been prepared and characterized based on their spectroscopic data. A distorted square-pyramidal structure has been proposed for the new complexes. The future work would be on the determination of in vitro antioxidant and free radical scavenging activities of these complexes using standard assays.
ACKNOWLEDGEMENTS
- The authors are grateful to the Western Illinois University Research Council and the US National Cancer Institute-NIH (AREA grant # 1R15 CA115404-01) for support.
References
| [1] | Snyder, S.H., 1976, Amer. J. Psychiatry, 133, 197 |
| [2] | O. Bratfos and J.O. Haug, Acta Psychiat, Scand., 60, 1, 1979 |
| [3] | A.R. Katritzky and A.J. Boulton (Eds.), Advances in heterocyclic Chemistry, Academic press, New York, 1968 |
| [4] | Keshavan, B., and Seetharamappa, J., 1987, Polyhedron, 6(3), 465 |
| [5] | Keshavan, B., and Seetharamappa, J., 1986, Synth. React. Met.-Org. Chem., 16(7), 979 |
| [6] | Keshavan, B., and Janardhan, R., 1987, Ind. J. Chem., 26A, 975 |
| [7] | Keshavan, B., and Janardhan, R., 1987, Ind. J. Chem., 25A, 1054 |
| [8] | Sanke Gowda, H., and Jayarama., 1981, J. Inorg. Nucl. Chem., 43(10), 2329 |
| [9] | Kroener, R., Heeg, M. J., and Deutsch, E., 1988, Inorg. Chem., 27, 558 |
| [10] | Made Gowda, N.M., and Phyu, H.P., 1992, Ttransition Met. Chem., 17, 467; H.P. Phyu, M.S. Thesis, Western Illinois University, Macomb, USA, May, 1991 |
| [11] | Made Gowda, N.M., Phyu, H.P., and Ackerson, B.E., 1993, Transition Met. Chem., 18, 64 |
| [12] | Made Gowda, N.M., Kyi, M.M., and Zhang, L., 1993, Transition Met.Chem., 18, 518; M.M. Kyi, MS. Thesis , Western Illinois University, Macomb, USA, December 1991; Made Gowda, N.M., and Zhang, L., 1994, Synth. React. Inorg. Met-Org. Chem., 24(5), 831; L. Zhang, M.S. Thesis, Western Illinois University, Macomb, USA, May, 1992 |
| [13] | Made Gowda, N.M., Ackerson, B.E., Morland, M., and Rangappa, K.S., 1993, Transition Met. Chem., 18, 271 |
| [14] | Made Gowda, N.M., Pacquette, H.L., Kim, D.H., and Jayaram, B., 1996, J. Mol. Struct., 382 ,129; Made Gowda, N.M., Vallabhaneni, R.K., Gajula, I., and AAFZAL, D., 1996, Synth. React Inorg. Met-Org. Chem, 26(4), 685 |
| [15] | Made Gowda, N.M., Lawrence Pacquette, H., Kim Doo-Hyung, Jayaram, Beby, 1996, J. Mol. Struct, 382, 129-135; Made Gowda, N.M., Rouch, W.D., and Viet, A.Q., 1993, The Chemistry of copper and Zinc Triads., Royal Society of Chemistry, Cambridge, U.K, 117-120 |
| [16] | Made Gowda, N.M., Vallabhaneni, R.K., Gajula, I., Ananda, S., 1997, J. Mol. Struct., 407, 125-130 |
| [17] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2011, Synthesis, characterization, and antioxidant activity evaluation of pyridoxine and its transition metal complexes., Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 41, 1-12 |
| [18] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2009, Synthesis, Characterization and Antioxidant Activity of Zinc(II) and Ruthenium(III) Pyridoxine Complexes., Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 39(8), 434-440 |
| [19] | Chaitanya Lakshmi G., Ananda S., and Made Gowda N.M., 2010, Synthesis of Iron-Pyridoxine Complex by Solvothermal Process, Its Structural Characterization and Antioxidant Activity Evaluation., J. Chem. Chemical. Engg, 4(12), 33-37 |
| [20] | J.M. Huheey, E.A. Keiter and R.L. Keiter, Inorganic Chemistry; Principles of Structures and Reactivity, 4th ed., Harper Collins College Publishers, 1993 |
| [21] | L.J. Bellemy, The Infrared Spectra of Complex Molecules, Methuen, London, p.355, 1964 |
| [22] | K. Nkamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley Interscience, New York, 1970 |
| [23] | D.A. Skoog and D.M. West, Principles of Instrumental Analysis, Saunders College, P.A, 1980, 171-173 |
| [24] | Jayarama, Thimmaiah, K.N., and D’Souza, M.V., 1985, J. Indian Chem. Soc., 62, 418 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML