-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(2): 29-31
doi: 10.5923/j.chemistry.20110102.06
L-Tryptophan, a Study on Interactions in Cu(II) Binary and Ternary Complexes in Aqueous Solution
S. A. A. Sajadi
Sharif University of Technology, Institute of Water & Energy, Tehran, P. O. Box 11155-8639, Iran
Correspondence to: S. A. A. Sajadi , Sharif University of Technology, Institute of Water & Energy, Tehran, P. O. Box 11155-8639, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The acidity and stability constants of M(Trp)1 M: Cu2+, Cu(Bpy2)2+, and Cu(Phen3)2+ complexes, were determined by potentiometric pH titration. It is shown that the stability of the binary Cu(Trp) complexes is determined by the basicity of the carboxylate group on one side and amino group on the other side. It is demonstrated that the equilibrium, Cu(Har4)2+ + Cu(Trp)  Cu(Har)(Trp) + Cu2+, is displacement due to the well known experience that mixed ligand complexes formed by a divalent 3d ion, a heteroaromatic N base and an O donor ligand possess increased stability. The other part of this displacement, which amount on average to an increased stability of the mixed ligand Cu(Bpy)(Trp) and Cu(Phen)(Trp) complexes of about 0.97 or 1.31 log unit. The stability constants of the 1:1 complexes formed between Cu2+, Cu(Bpy)2+ or Cu(Phen)2+ and Trp2−, were determined by potentiometric pH titration in aqueous solution (I = 0.1 M, NaNO3, 25℃). The order of the stability constants was reported.
Cu(Har)(Trp) + Cu2+, is displacement due to the well known experience that mixed ligand complexes formed by a divalent 3d ion, a heteroaromatic N base and an O donor ligand possess increased stability. The other part of this displacement, which amount on average to an increased stability of the mixed ligand Cu(Bpy)(Trp) and Cu(Phen)(Trp) complexes of about 0.97 or 1.31 log unit. The stability constants of the 1:1 complexes formed between Cu2+, Cu(Bpy)2+ or Cu(Phen)2+ and Trp2−, were determined by potentiometric pH titration in aqueous solution (I = 0.1 M, NaNO3, 25℃). The order of the stability constants was reported.
Keywords: Tryptophan, Divalent Metal Ions, Potentiometric Titration, Acidity and Stability Constants
Cite this paper: S. A. A. Sajadi , "L-Tryptophan, a Study on Interactions in Cu(II) Binary and Ternary Complexes in Aqueous Solution", American Journal of Chemistry, Vol. 1 No. 2, 2011, pp. 29-31. doi: 10.5923/j.chemistry.20110102.06.
Article Outline
1. Introduction
- L-Trp or D-Trp; sold for medical use as Tryptan (fig. 1)[1] is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG. Tryptophan (Trp) is considered exceptional in its diversity of biological functions[2]. It is a vital constituent of proteins and indispensable in human nutrition for establishing and maintaining a positive nitrogen balance[3]. Besides, some of its derivatives are potent drugs[4]. Trp is widely used in food industry. It is sometimes added to dietary and feed products as a food fortifier in order to maintain the amino acid balance of the food and correct possible dietary deficiencies. Trp can also be used to study structure and dynamics of the proteins because of its indole moiety[5]. In particular, Trp is the precursor of the neurotransmitter serotonin and plays an important role in brain function and related regulatory mechanisms[6]. In addition, Trp is an important and frequently used starting material in the chemical synthesis of a range of pharmaceuticals[7]. The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as demonstrated by its growth effects on rats. Now it is interesting to investigate the complex bilding of ternary systems with Trp. We would like to determine thethermodynamic constants of ternary complexes such as Cu(Har)(Trp).
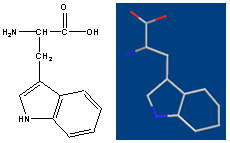 | Figure 1. Chemical formula of L-Tryptophan |
2. Experimental
2.1. Materials
- Chemicals were purchased from Merck. Copper(II) nitrate trihydrated, sodium nitrate, potassium hydrogen phthalate and standard solutions of sodium hydroxide (titrasol), 2,2'- bipyridyl, 1,10-phenanthroline, nitric acid, EDTA and of the buffer solutions of pH 4.0, 7.0 and 9.0 were from Merck. All the starting materials were pro analysis and used without further purification. Water was purified by Mili-Q water purification system, deionized and distillated.
2.2. pH titrations
- Reagents: Carbonate-free sodium hydroxide 0.03 M was preparated and standardized against sodium hydrogen phthalate and a standard solution of nitric acid 0.5 mM. Copper (II) nitrate solution (0.03 M) was prepared by dissolving the above substance in water and was standardized with standard solution of EDTA 0.1 M (triplex).
2.3. Apparatus
- All pH titrations was performed using a Metrohm 794 basic automatic titrator (Titrino), coupled with a Hero thermostating bath at 25℃ (±0.1℃) and a Metrohm combined glass electrode (Ag/AgCl). The pH meter was calibrated with Merck standard buffer solutions (4.0, 7.0 and 9.0).
2.4. Procedure
- For the determination of acid dissociation constants of the ligand Trp an aqueous solution (0.3 mM) of the protonated ligand was titrated with 0.03 M NaOH at 25℃ under nitrogen atmosphere and ionic strength of 0.1 M, NaNO3. For the determination of binary (one ligand and Cu2+) and ternary systems (Cu2+, one of the other L ligand (Har) and Trp), the ratios used were 1:1, 1:2 Cu(II) : ligand and 1:1:1, Cu(II) : Trp : Har, 0.3 mM. This solution was titrated with 0.03 M NaOH under the same conditions mentioned above. Each titration was repeated seven times in order to check the reproducibility of the data. CalculationThe acid dissociation constants,
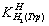 and
and 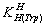 for H2(Trp) were calculated by an algebraic method. The equilibria involved in the formation of 1:1 complex of Trp and a divalent metal ion may be expressed as equations (3) & (4).
for H2(Trp) were calculated by an algebraic method. The equilibria involved in the formation of 1:1 complex of Trp and a divalent metal ion may be expressed as equations (3) & (4).3. Results and Discussion
- In this section we would discuss the resulted data.
3.1. Acidity Constants
- Tryptophan (Trp) can accept one proton on carboxylic group, for which the following deprotonation equilibria hold:
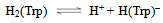 | (1a) |
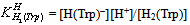 | (1b) |
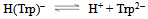 | (2a) |
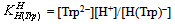 | (2b) |
3.2. Stability of Binary and Ternary Complexes
- If we abbreviate for simplicity Cu2+, Cu(Bpy)2+, and Cu(Phen)2+ with M2+, one may write the following two equilibria (3) & (4):
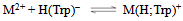 | (3a) |
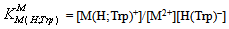 | (3b) |
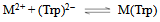 | (4a) |
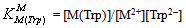 | (4b) |
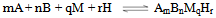 | (5a) |
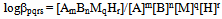 | (5b) |
 | (6a) |
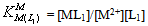 | (6b) |
 | (7a) |
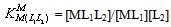 | (7b) |
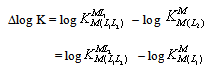 | (8) |
3.3. Potentiometric Analyses
- The model of species for this ternary system that was used in superquad program includes all the species of table 1 as well as the hydrolysis of Cu2+[11,12]. The stability constants of the binary complexes were refined separately using the titration data of this system in a 1:1 and 1:2 ligand:Cu2+ ratio in the same conditions of temperature and ionic strength. They were fixed and, consequently, only ternary species were refined in ternary model of the species. The results are summarized in Table 1.
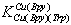 5.441 kJ/mol and for Δlog
5.441 kJ/mol and for Δlog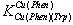 7.349 kJ/mol, which are considerable high. This means that interaction between Cu(Har)2+ and trp2− is relative strong and the observed increased stability indicate strong complex bilding of ternary systems.
7.349 kJ/mol, which are considerable high. This means that interaction between Cu(Har)2+ and trp2− is relative strong and the observed increased stability indicate strong complex bilding of ternary systems.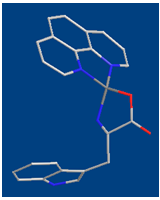 | Figure 2. Schematic structures of the species with interactions according to equilibrium (4) & (7) for Cu(Phen)(Trp). The structure in the right part of the figure was drawn with the program CS Chem 3D, version 3.5, from Cambridge Software Corporation |
Notes
- 1. Trp: L-Tryptophan 2. Bpy: 2,2'-Bipyridyl3. Phen: 1,10-phenanthroline4. Har: Heteroaromatic ligand such as Bpy or Phen
References
| [1] | IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. |
| [2] | http://www.chem.qmul.ac.uk/iupac/AminoAcid/. Retrieved 2007-05-17. Gollnick P, Babitzke P, Antson A, Yanofsky C, 2005, "Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis". Annu. Rev. Genet. 39: 47–68 |
| [3] | Moffat, A.C., Jackson, J.V., Moss, M.S., Widdop, B., 1986: Clarke’s Isolation and Identification of Drugs, The Pharmaceutical Press, London, UK p. 1056 |
| [4] | Fiorucci, A.R., Cavalheiro, E.T.G., 2002, The use of carbon paste electrode in the direct voltammetric determination of tryptophan in pharmaceutical formulations, J. Pharm. Biomed. Anal. 28, 909–915 |
| [5] | H.H. Hussey, Sleep inducement by L-tryptophan, J. Am. Chem. Soc. 87, 1126, (1974) |
| [6] | Cioni, P., Strambini, G.B., 2002, Tryptophan phosphores- cence and pressure effects on protein structure, Biochim. Biophys. Acta, 1595, 116–130 |
| [7] | Liang, Y.D., Song, J.F., 2005, Flow-injection chemilumin- escence determination of tryptophan through its peroxidation and epoxidation by peroxynitrous acid, J. Pharm. Biomed. Anal.38, 100–106 |
| [8] | Altria, K.D., Harkin, P., Hindson, M.G., 1996, Quantitative determination of tryptophan enantiomers by capillary electrophoresis, J. Chromatogr. Biomed. 686, 103–110 |
| [9] | Handbook of Chem. & Physics, 1975, 55 , p.129 |
| [10] | Miranda J.L, Felcman J, 2003, Polyhedron, 22 ; p. 225-233 |
| [11] | Sigel H, 1973, Metal Ions in Biological Systems, Mixed Ligand Complexes, Marcel Dekker, New York, vol.2; p. 3 |
| [12] | Felcman J, Miranda J.L, 1997, J. Braz. Chem. Soc., 8 ; p. 575 |
| [13] | Pettit L.D, Powel A., 1998, IUPAC Stability Conatants Database, Release 3, version 3.02, Academic Software Timble, UK. Sigel H., Zuberbuehler A.D., Yamauchi O. 1991, Anal. Chim. Acta, 63, p. 255 |
| [14] | Sigel H., Naumann C.F., 1976, J. Am. Chem. Soc., 98(3), 730-739 |
| [15] | Sajadi, S.A. A., Song, B., Sigel, H., 1998, Inorg. Chim. Acta, 283, 193-201 |
| [16] | Sajadi, S. A. A., Song, B., Gregan, F., Sigel, H., 1999, Inorg. Chem., 38(3), 439-448 |
| [17] | Sajadi, S. A. A., Song, B, Gregan, F., Sigel, H., 1997, Bull. Chem. Soc. Ethiop., 11(2), 121-130 |
| [18] | Sajadi, S. A. A., Bastian, M., Sigel, H., 1995, J. Inorg. Biochem., 59(2,3), 139 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML