-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(1): 22-25
doi: 10.5923/j.chemistry.20110101.04
Vanadium Substituted H7SiW9V3O40 as a Versatile Catalyst for Dakin–West Synthesis of Acetamido Carbonyl Compounds
Reza Tayebee , Shima Taizabi
Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran
Correspondence to: Reza Tayebee , Department of Chemistry, School of Sciences, Sabzevar Tarbiat Moallem University, Sabzevar, 96179-76487, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Four-component condensation of an aromatic aldehyde, acetonitrile, acetyl chloride, and an enolisable ketone in the presence of H7SiW9V3O40catalyst is investigated. Findings revealed a very good catalytic activity of H7SiW9V3O40 in the desired condensation reaction. The results revealed that H7SiW9V3O40 was effective, inexpensive, recyclable, and eco-friendly.
Keywords: Four-component, Acetamido Ketone, Keggin, Heteropolyacid
Cite this paper: Reza Tayebee , Shima Taizabi , "Vanadium Substituted H7SiW9V3O40 as a Versatile Catalyst for Dakin–West Synthesis of Acetamido Carbonyl Compounds", American Journal of Chemistry, Vol. 1 No. 1, 2011, pp. 22-25. doi: 10.5923/j.chemistry.20110101.04.
1. Introduction
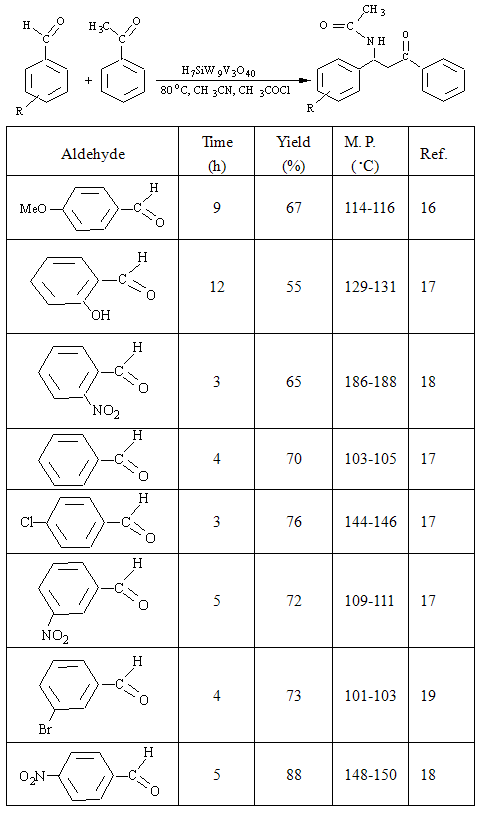
- The design of multi-component reactions is an important field of research and there has been tremendous developments in three- and four-component reactions. These protocols are performed without isolation of any intermediates, thus reducing time and saving both energy and raw materials. β-Acetamido ketones are important compounds which have been used in the synthesis of other important organic molecules such as 1,3-amino alcohols, nucleoside peptide antibiotics, nikkomycines, and neopolyoxins[1, 2]. The best known route for the synthesis of this important class of compounds is proposed as Dakin–West reaction[3]. This reaction involves the one-pot multicomponent coupling condensation of an enolisable ketone, aldehyde and acetonitrile in the presence of acetyl chloride[4]. A few catalysts have already been applied to synthesis of acetamido ketones such as CoCl2[5], montmorillonite K10 clay[6], silica sulphuric acid[7], Cu(OTf)2, transition metal triflates, various metal chlorides[8] and solid acid Hβ-zeolite[9]. However, most of these procedures are not entirely satisfactory and suffer from long reaction time or tedious work up. Hence, the development of new catalysts with more efficiency is of interest[10].Herein, we developed the applicability of strong super-acidic vanadium(V)-substituted Keggin-type heteropolyacid, H7SiW9V3O40, as catalyst for the efficient and facile synthesis of β-acetamido ketones through one-pot condensation of an aryl aldehyde, acetophenone, acetyl chloride, and acetonitrile (Scheme 1).
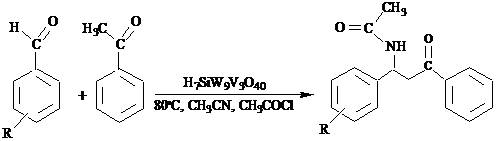 | Scheme 1. |
2. Experimental
- All starting materials were purchased commercially and were used as received. All products were characterized by comparison of their spectral and physical data with those reported in the literature. Silica gel 60 (70—230 mesh) was used for column chromatography. Progress of the reactions was monitored by TLC. Infrared spectra were recorded (KBr pellets) on a 8700 Shimadzu Fourier Transform spectrophotometer. 1HNMR spectra were recorded on a Bruker AVANCE 300-MHz instrument. The catalyst was prepared and characterized according to literature procedures[11-14].H7SiW9V3O40 was prepared from sodium vanadates precursor. Sodium vanadate (1.9 g; 15.5 mmol) was dissolved in 300 ml of water. Na10[α-SiW9O34].18H2O (145 g; 52 mmol) was added to the stirred solution, followed by 185 ml of 6 M sulfuric acid. The solution then was maintained under stirring for 45 min. The pH was adjusted between 6 and 7 by the addition of solid potassium carbonate. An orange potassium salt (∼100 g) was precipitated via the addition of solid potassium chloride (80 g) and recrystallized in water. Calcd (Found): K, 9.34 (9.12); Si, 0.96 (1.12); V, 5.16 (5.28); W, 56.48 (56.73); H2O, 6.14 (6.32).FT-IR (cm-1): 1004(w), 960(s), 900(vs), 805(vs), 740(vs). Then, the potassium salt (15 g; 5 mmol) was dissolved in 65 ml of water, and the solution was placed in a separatory funnel. Diethyl ether then was added, followed by the slow addition of concentrated hydrochloric acid (100 ml). The heavy phase was collected and diethyl ether evaporated under vacuum. The resultant solid was dissolved in a minimum amount of water. Finally, the heteropolyacid was slowly crystallized at room temperature.A mixture of aromatic aldehyde (1 mmol), acetophenone (1 mmol), acetyl chloride (2 mmol) in acetonitrle (4 ml) was treated with a catalytic amount of the desired heteropolyacid at 80 ◦C. Progress of the reaction was monitored by TLC. The work-up procedure of this reaction is very simple. After completion of the reaction, the mixture was filtered to separate the catalyst. Then, the solid crude product was washed with petroleum ether and filtered. The pure product, if needed, could be obtained by re-crystallization from ethanol-water mixture. All products were identified by means of IR and 1H NMR spectroscopy and/or comparison of their melting points with those reported in the literature.
3. Results and Discussion
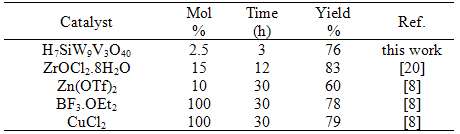
- The catalytic proficiency of the corresponding heteropolyacid in the four-component condensation of para- chloro-benzaldehyde with acetophenone and acetyl chloride in refluxing acetonitril was studied. Findings revealed that Keggin H7SiW9V3O40 behaved as active catalyst, due to its bi-functional nature originating from their strong acidic protons and presence of vanadium (V) transition metal ion, as effective electron acceptor resource[15]. The four-component coupling condensation of benzaldehyde, acetophenone, and acetyl chloride was performed in acetonitrile in the absence of catalyst. Findings revealed that the reaction could not be productive, and no reaction was happened even after prolonged reaction time (10 h), indicating that this is indeed a heteropolyacid catalyzed reaction. At first, the four-component coupling condensation of benzaldehyde, acetophenone, and acetyl chloride was performed in acetonitrile in the absence of catalyst. Findings revealed that the reaction could not be productive, and no reaction was happened even after prolonged reaction time (10 h), indicating that this is indeed a heteropolyacid catalyzed reaction (Table 1). The catalytic activity and efficacy of the present method can be influenced by miscellaneous parameters such as kind and amount of the employed catalyst, solvent system, and temperature. To establish the optimal reaction condition, a set of experiments varying the amount of the catalyst, quantity of acetyl chloride, and temperature were carried out. Initially, different mol% of H7SiW9V3O40 was taken into account (Table 1). The best condition to prepare the β-acetamido ketones was achieved when 2.5 mol% of vanadium(V)-containing heteropolyacid, H7SiW9V3O40, was accomplished (Table 1). 4-chlorobenzaldehyde led to 76% of product after 3 h); whereas, higher mol% of catalyst (10 mol%) resulted in 82% conversion in short time 0.5 h. Employing smaller amounts of the catalyst (0.5 mol%) diminished the product yield to 53% after 6 h.
|
|
|
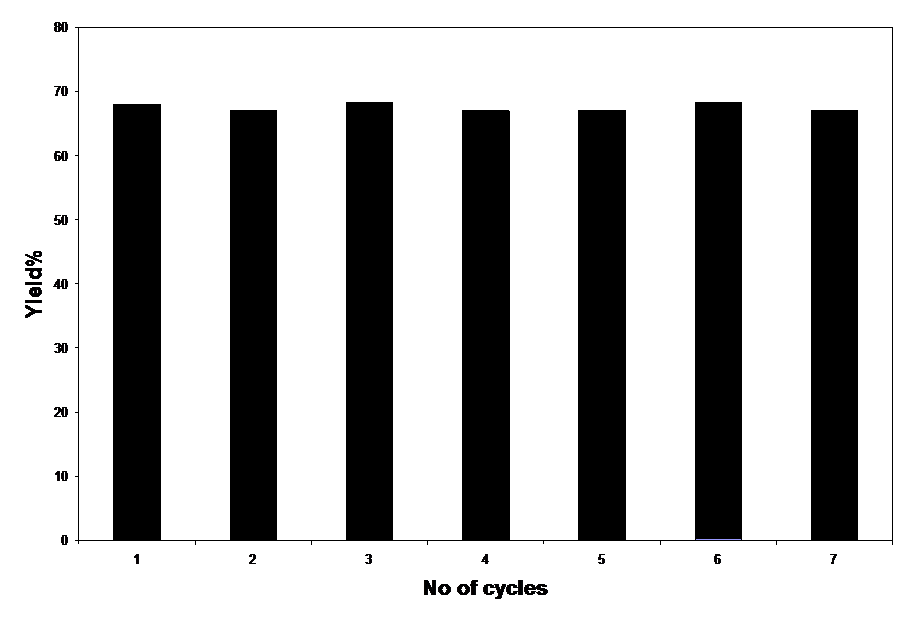 | Figure 1. Studying Reusability of H7SiW9V3O40. |
4. Conclusions
- In conclusion, this report illustrated a new convenient and efficient method for the preparation of a wide range of β-acetamido ketones by using H7SiW9V3O40. The present methodology offers attractive features, such as easy workup, simple experimental procedure, relatively short reaction times, high yields, and using a recyclable catalyst which will have wide scope in organic synthesis.
References
| [1] | A. G. Godfrey, D. A. Brooks, L. A. Hay, M. Peters, J. R. McCarthy, and D. Mitchell, 2003, Application of the Dakin−West reaction for the synthesis of oxazole-containing dual PPARα/γ agonists., J. Org. Chem. 68, 2623 |
| [2] | K. Kobinata, M. Uramoto, M. Nishii, H. Kusakabe, G. Nakamura, and K. Isono, 1980, Agric. Biol. Chem. 44, 1709 |
| [3] | H. D. Dakin, and R. West, 1982, NaHSO4.H2O as an efficient and eco-friendly catalyst for the one-pot multicomponent synthesis of β-acetamido ketones under mild and heterogeneous conditions. J. Biol. Chem. 78, 745 |
| [4] | B. Bhatia, M. M. Reddy, and J. Iqbal, 1994, Cobalt-catalyzed three-component coupling involving ketones or ketoesters, aldehydes and acetonitrile: A novel one-pot synthesis of β-acetamido ketones. J. Chem. Soc. Chem. Commun. 713 |
| [5] | M. Mukhopadhyay, B. Bhatia, and J. Iqbal, 1997, Synthesis of β-amino carbonyl compounds via the iodine-alumina catalyzed three-component coupling reaction under microwave irradiation. Tetrahedron Lett. 38, 1083 |
| [6] | D. Bahulayan, S. K. Das, and J. Iqbal, 2003, Montmorillonite K10 clay: An efficient catalyst for the one-pot stereoselective synthesis of β-acetamido ketones. J. Org. Chem. 68 5735 An efficient catalyst for the one-pot stereoselective synthesis of β-acetamido ketones. J. Org. Chem. 68 5735 |
| [7] | M. M. Khodaei, A. R. Khosropour, and P. Fattahpour, 2005, A modification procedure for the Dakin-West reaction: An efficient and convenient method for a one-pot synthesis of β-acetamido ketones using silica sulfuric acid as catalyst. Tetrahedron Lett. 46, 2105 |
| [8] | G. Pandey, R.P. Singh, A. Garg, and V.K. Singh, 2005, Synthesis of Mannich type products via a three-component coupling reaction. Tetrahedron Lett. 46, 2137 |
| [9] | R.P. Bhat, P.R. Vivek, M.A. Varghese, B.P. Sachin, and S.D. Samant, 2005, SnCl2·2H2O-catalyzed efficient synthesis of β-acetamido ketones and β-acetamido ketoesters under solvent-free conditions. Tetrahedron Lett. 46, 4801 |
| [10] | R. Singh, R.M. Kissling, M. A. Letellier, and S. P. Nolan, 2004, Trans-esterification/ acylation of secondary alcohols mediated by N-heterocyclic carbene catalysts. J. Org. Chem. 69, 209 |
| [11] | G. A. Tsigdinos, and C. Hallada, 1968, Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis. J. Inorg. Chem. 7, 437 |
| [12] | S. Farhadi, and M. Taherimehr, 2008, Decatungstodivanadogermanic heteropoly acid (H6GeW10V2O40.22H2O): A novel, green and reusable catalyst for efficient acetylation of alcohols and phenols under solvent-free conditions. Acta Chim. Slov. 55, 637 |
| [13] | E. Cadot, R. Thouvenot, A. Teze, and G. Herve, 1992, Organophosphoryl derivatives of trivacant tungstophosphates of general formula α-A-[PW9O34(RPO)2]5–: synthesis and structure determination by multinuclear magnetic resonance spectroscopy (31P, 183W). Inorg. Chem. 31, 4128 |
| [14] | M. H. Alizadeh, S. P. Harmalker, Y. Jeanin, and M. T. Pope, 1985, A new supramolecular assembly based on triple-Dawson-type polyoxometalate and 3d-4f heterometallic cluster. J. Am. Chem. Soc. 107, 2662 |
| [15] | C. Marchal-Roch, and J.-M. Millet, 2001, Study of Te and V as counter-cations in Keggin type phosphomolybdic polyoxometalate catalysts for isobutane oxidation. C. R. Acad. Sci. Paris Sér. Chim. 4, 321 |
| [16] | V. Siddaiah, G. L. V. Damu, D. Sudhakar, C. Venkata Rao, and V. Christopher, 2008, VCl3 Catalyzed, a Simple and efficient one-pot, multi-component synthesis of β-acetamido carbonyl compounds. J. Kor. Chem. Soc. 52, 712 |
| [17] | E. Rafiee, F. Tork, and M. Joshaghani, 2006, Heteropoly acids as solid green Brønsted acids for a one-pot synthesis of β-acetamido ketones by Dakin–West reaction. Med. Chem. 16, 1221 |
| [18] | T. Yakaiah, B. P. V. Lingaiah, G. Venkat Reddy, B. Narsaiah, and P. Shanthan Rao, 2007, Perfluorinated resin-sulfonic acid (Nafion-H): an efficient, environment friendly and recyclable heterogeneous catalyst for the one-pot multicomponent synthesis of β-acetamido ketones. ARKIVOC (xiii), 227 |
| [19] | A. Davoodnia, M. M. Heravi, L. Rezaei-Daghigh, and N. Tavakoli-Hoseini, 2009, Imidazolium-based zwitterionic butane-1-sulfonates: Synthesis and properties of 4-(1- (2-Cyanoethyl) imidazolium)butane-1-sulfonate and crystal structures of 4-(1-Alkylimidazolium)butane-1- sulfonates (Alkyl = Methyl, Ethyl, Propyl). Monat. Chem. 140, 1499 |
| [20] | R. Ghosh, S. Maiti, A. Chakraborty, S. Chakraborty, and A. K. Mukherjee, 2006, ZrOCl2·8H2O: An efficient Lewis acid catalyst for the one-pot multicomponent synthesis of β-acetamido ketones. Tetrahedron 62, 4059 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML