-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2011; 1(1): 16-21
doi: 10.5923/j.chemistry.20110101.03
Invertase Immobilization on a Metal Chelated Triazole-Functionalized Eupergit® C
K. Uzun , E. Çevik, M. Şenel
Department of Chemistry, Faculty of Arts and Sciences, Fatih University, B. Cekmece, Istanbul, 34500, Turkey
Correspondence to: E. Çevik, M. Şenel , Department of Chemistry, Faculty of Arts and Sciences, Fatih University, B. Cekmece, Istanbul, 34500, Turkey.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the present study, Eupergit® C macroporous beads were functionalized with amino triazole. Cu2+ ions were chelated on the triazole modified Eupergit® C (EC), and subsequently, the metal chelated beads were used in the adsorption of invertase. The maximum reaction rate (Vmax) and the Michaelis-Menten constant (Km) were determined for the free and the immobilized enzymes. The characteristics of immobilized invertase such as the temperature activity curve, thermal stability, operational stability, and storage stability were evaluated. The results indicated that triazole functionalized Eupergit® C beads were well applicable for metal adsorption and enzyme immobilization.
Keywords: Invertase; Immobilization; Eupergit® C; Triazole; Chelating Beads, Enzyme
Cite this paper: K. Uzun , E. Çevik, M. Şenel , "Invertase Immobilization on a Metal Chelated Triazole-Functionalized Eupergit® C", American Journal of Chemistry, Vol. 1 No. 1, 2011, pp. 16-21. doi: 10.5923/j.chemistry.20110101.03.
Article Outline
1. Introduction
- The employment of free enzymes in biotechnological applications is limited owing to the high cost and the impracticality of recovering and separating the enzymes from the reaction medium at the end of the enzymatic pr˚Cess. In order to overcome these difficulties, enzymes were immobilized to obtain a catalyst that would be separable from the reaction medium. Furthermore, the immobilization would provide the enzymes with good properties such as resistance to temperature and denaturants, as well as a longer stability as compared to their free counterparts[7]. Until now, different methods have been developed for enzyme immobilization. Some examples of such methods are cross-linking, covalent attachments, adsorption and entrapment/encapsulation of biological molecules within polymeric gels or carbon paste[8, 13, 1, 4]. Among these methods, the adsorption to a solid support material is the oldest prot˚Col of the physical adsorption method; this method is the most commonly used since it is easiest to perform. The most significant advantages of this method are the stability of enzyme activity after immobilization, and the ability of reuse of the enzyme and support material for different purposes owing to the reversibility of the method. Reversible enzyme immobilization is a very powerful tool that may be considered for solving the cost problem associated with the current methods. Reversible immobilezation provides the possibility of using such enzymes in an immobilized form, thereby providing the advantages of both using immobilized enzymes as well as saving time and cost[12] S. Akgöl and A. Denizli, J. Mol. Catal. B: Enzym. 28 (2004), p. 7.[14, 5].Eupergit® C, which is comprised of epoxy-activated bead polymers formed from a hydrophilic acrylamide with ally glycidyl (epoxide) groups as the active components responsible for binding, is commonly used for enzyme immobilization through different functional groups (amino, sulfhydryl, hydroxyl, phenolic ones) of enzymes[6]. Invertase catalyzes the hydrolysis of sucrose to glucose and fructose, and is known as the invert sugar. However, sucrose crystallizes more readily than invert sugar, and therefore, the latter is widely used in the production of non-crystallizing creams, for example in making jam and artificial honey[11] J.S. Melo and S.F. D'Souza, J. Bi˚Chem. Biophys. Methods 42 (2000), p. 133. Article | PDF (61 K) | View Record in Scopus | Cited By in Scopus (11)[10]. Despite the fact that invertase has little potential for commercial use in its immobilized form, it is still considered to be one of the most studied enzymes since it is a model enzyme for experimental purposes[15].In the present study, triazole functionalized Eupergit® C beads were prepared, chelated with Cu2+ ions and used for the invertase immobilization. Cu2+ ions coordinate with the triazole groups of the Eupergit C®-Tri (EC-Tri) beads and the enzyme binds the beads via chelated metal ion. The protein adsorption capacity, coupling efficiency, Michaelis-Menten kinetic constants (Km and Vmax), and the reuse and the storage stability of the free and the immobilized enzymes were evaluated.
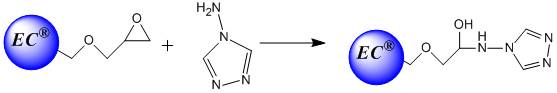 | Figure 1. Modification of Eupergit C® with aminotriazole. |
2. Materials and Methods
2.1. Materials
- Invertase (β-fructofuranosidase) type V (EC No.3.2.1.26) was purchased from Sigma and used as received without further purification. Eupergit C® was kindly donated by Röhme GmbH & Co., Degussa (Darmstadt, Germany). 4-amino-1,2,4-triazole was obtained from Fluka. All other chemicals were of analytical grade and were used without further purification.
2.2. Modification of Eupergit C® and Adsorption of Invertase
- Eupergit C® is a spherical acrylic polymer (having particles with diameter of 150 µm) containing epoxide groups as reactive components. These groups function as active components for the covalent binding of ligands containing amino, mercapto, or hydroxyl groups. Eupergit C®-Tri (EC®-Tri) beads were prepared by the conventional method on Eupergit C® supports which involved direct triazole binding on polymers via oxirane groups as shown in Figure 1. Eupergit C® was incubated with an excess amount of triazole in 1.0 M phosphate buffer solution (pH 7.0) at 25˚C. After incubation for 4 days, the beads were collected on a sintered-glass filter, washed with 1.0 M NaCl for three times, and subsequently washed with the same buffer solution. The excess of epoxide groups on the Eupergit C®-Tri beads were bl˚Cked by incubation with glycerol for 4 h at 25˚C.Chelates of Cu2+ ions with EC®-Tri beads were prepared by mixing 0.1 g of beads with 5 ml aqueous solutions containing 50 ppm Cu2+ ions at a constant pH value of 5.0 (adjusted with HCl and NaOH). The flask was shaken at 25℃ for 24 h. The concentration of Cu2+ ions in the resulting solution was determined using a graphite furnace atomic adsorption spectrometer (Analyst 800/Perkin Elmer, USA). The amount of adsorbed Cu2+ ions was calculated by determining the concentrations of the Cu2+ ions in the initial solution and in the equilibrium. Invertase was adsorbed onto the Cu2+ chelated Eupergit C®-Tri beads at various pH values, either in acetate buffer (0.1 M, pH 3.0-5.5) or in phosphate buffer (0.1 M, pH 6.0-9.0). The initial invertase concentration was determined as 1 mg/ml. The adsorption experiments were conducted for 3 h at 25˚C while shaking continuously. At the end of the time period, the enzyme adsorbed beads were removed and washed three times with the same buffer solution. The enzyme adsorbed beads were then stored at 4˚C in fresh buffer solution until use. The amount of adsorbed invertase (Q) was calculated using the following equation:
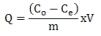 where, Q is the amount of adsorbed invertase (mg protein/g Eupergit C®-Tri beads); Co and Ce are the initial and equilibrium concentrations in the solution (mg/ml); V is the solution volume (ml); and m is the mass of the Eupergit C®-Tri beads (g).
where, Q is the amount of adsorbed invertase (mg protein/g Eupergit C®-Tri beads); Co and Ce are the initial and equilibrium concentrations in the solution (mg/ml); V is the solution volume (ml); and m is the mass of the Eupergit C®-Tri beads (g).2.3. Invertase Desorption Studies
- The invertase adsorption and desorption cycle was repeated 10 times in order to evaluate the reusability of EC®-Tri-Cu2+ beads. Invertase desorption from EC®-Tri-Cu2+ beads was carried out with 25 mM EDTA. The beads were washed several times with phosphate buffer (0.1 M, pH 7.0), and were subsequently reused in enzyme immobilization.
2.4. Enzyme and Protein Assay
- The enzymatic activities of the immobilized and freeinvertasewere determined by Nelson’s method[11]. Various concentrations of sucrose solution were preincubated for 10 min at 25°C, and the enzyme electrode was placed in sucrose solutions for specific reaction times (2, 4, and 6 min). After removing the immobilized enzymes, 1 mL aliquots were drawn and added to 1 mL Nelson’s reagent to terminate the reaction. Subsequently, the tubes were placed in a boiling water bath for 20 min. The tubes were then cooled down and 1 mL of arsenomolybdate reagent was added. As a final step, 7 mL of distilled water was added to each test tube and mixed by vortexing. Once the mixing was complete, the absorbances for the blank and the substrate solutions were determined at 540 nm using a double beam spectrophotometer. One unit of invertase activity was defined as the amount of enzyme required to release 1 μmol glucose from sucrose per minute at a pH value of 5 and a temperature of 25°C.The protein concentrations of polymer networks were determined by Lowry method[9] using bovine serum albumin as the standard. The quantity of entrapped protein was calculated by subtracting the amount of protein recovered in the combined washings of the invertase-EC from the amount of protein used for immobilization.
3. Properties of Free and Immobilized Enzymes
3.1. Optimum PH
- The effect of pH on the activity and the stability (incubated for two hours in buffer solution) of the free and the immobilized invertase was assayed in acetate buffer (0.1 M) in the pH range of 3.0-6.5 and in phosphate buffer (0.1 M) in the pH range of 7.0-9.0 using the standard activity assay pr˚Cedure mentioned above.
3.2. Optimum Temperature
- The effect of temperature and thermal stability on the activity of both free invertase and bound invertase was studied in the temperature range of 20˚C to 80˚C and assayed under the standard assay conditions. The thermal stability of invertase was evaluated by measuring the residual activity of invertase exposed to various temperatures ranging between 20˚C and 80˚C in phosphate buffer (0.1 M, pH 7.5) for 15 minutes. After heating, the samples were quickly cooled to 25˚C and assayed immediately for determining the enzymatic activity as done earlier.
3.3. Storage Stability and Reusability
- The immobilized invertase was stored in phosphate buffer (0.1 M, pH 7.5) at 4 C and 25˚C for 35 days and its activity was measured at frequent intervals. The immobilized invertase was repeatedly used in order to test its reusability under the standard assay conditions. After each activity assay, the samples were washed with the buffer and then stored until the next assay in the buffer solution.
4. Results and Discussion
4.1. Adsorption of Invertase
- The adsorption of invertase for Cu2+-chelated triazole functionalized Eupergit® C beads is presented in Figure 2. The adsorption could ˚Ccur for specific interactions between invertase and the Cu2+ chelated EC-Tri beads. It can be observed from the graph that the adsorption efficiency is related to the concentration of the invertase in the adsorption. However, this was found to level off at an invertase concentration of 0, 8 mg/ml and the maximum adsorption for beads was obtained as 72, 8 mg/g. In addition, it can be observed that the adsorption could not be achieved for EC-Tri beads without Cu2+ chelation. These results indicate that copper chelation is important for the adsorption of enzyme[12]
4.2. Kinetic Parameters
- The kinetic parameters, Michaelis constants Km and Vmax for free and immobilized invertase were determined using the Lineweaver–Burk plot. For the free enzyme, Km was found to be 65.7 mM whereas Vmax was calculated as 153.2 µM min-1 mg-1enzyme. The Km value was found to be 27.6 mM for EC-Tri–Cu2+-invertase. The Vmax value of immobilized invertase was estimated from the data as 45.2 µM min-1 mg-1enzyme. As expected, the Km and Vmax values were significantly affected after adsorption onto the Cu2+ incorporated EC-Tri beads.
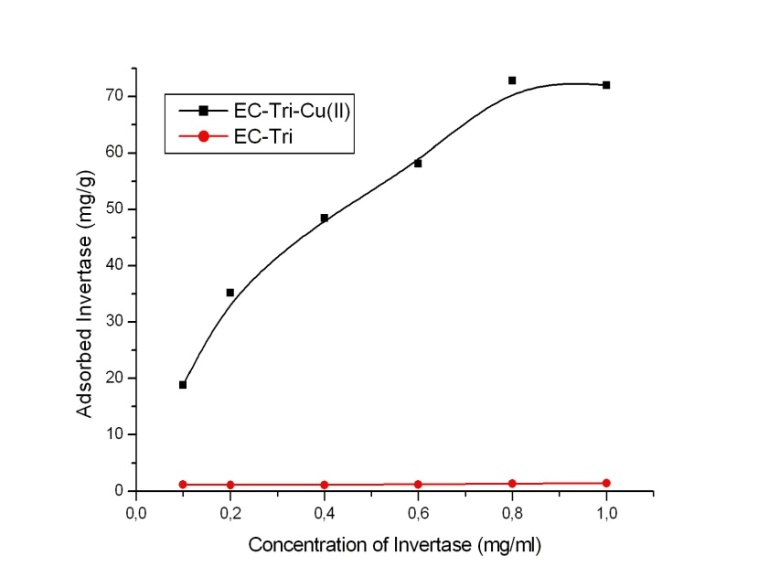 | Figure 2. Effect of invertase concentration on the adsorption efficiency of EC-Tri –Cu2+ |
4.3. Optimum Temperature
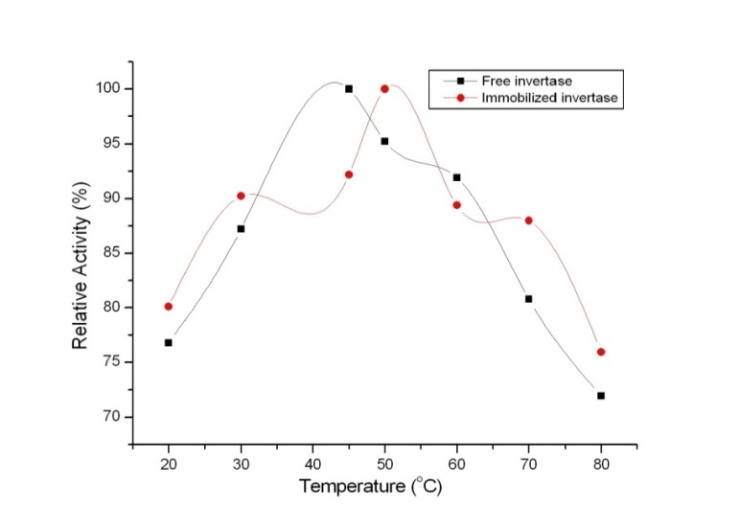 | Figure 3. Optimum temperature of the free and immobilized invertase |
4.4. Optimum PH
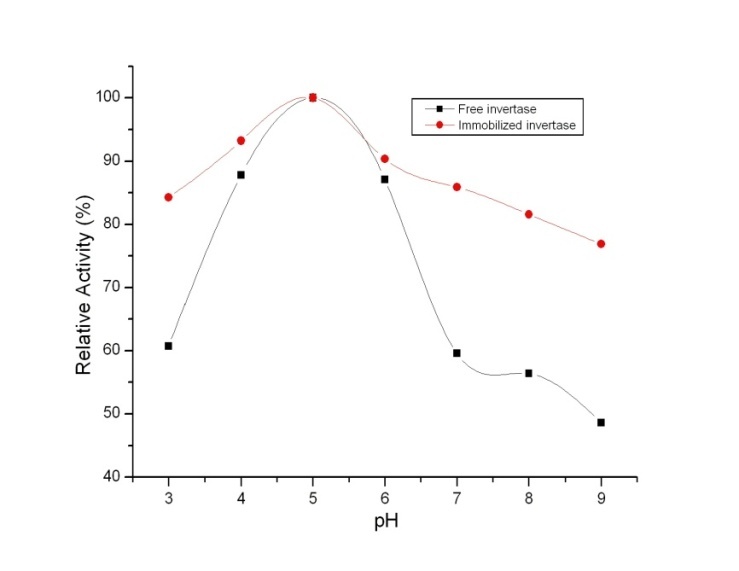 | Figure 4. pH profiles of free and immobilized invertase |
4.5. Storage Stability
- Another significant feature of immobilized enzymes is their storage stability which is an advantage when compared with free enzymes which tend to lose their activities considerably quickly (Figure 5). In general, if an enzyme is in aqueous solution, it is not stable during storage, and the activity is gradually decreased. The free invertase and the immobilized invertase were stored in pH 5.0 acetate buffer at 4˚C and 25˚C. The activities were then measured over a period of 35 days (Figure 6). It was found that for 25˚C, the free enzyme lost its 25% activity, and the immobilized enzyme lost about 22% of its activity. On the other hand, for 4 ˚C, the free enzyme lost about 19% of its activity whereas the immobilized enzyme lost about 10% of its activity during this period. This decrease in enzyme activity was explained as a time-dependent natural loss in enzyme activity; however, this could be prevented to a significant degree upon adsorption. The result clearly indicates that the immobilized invertase exhibits an improved stability over that of the free enzyme.
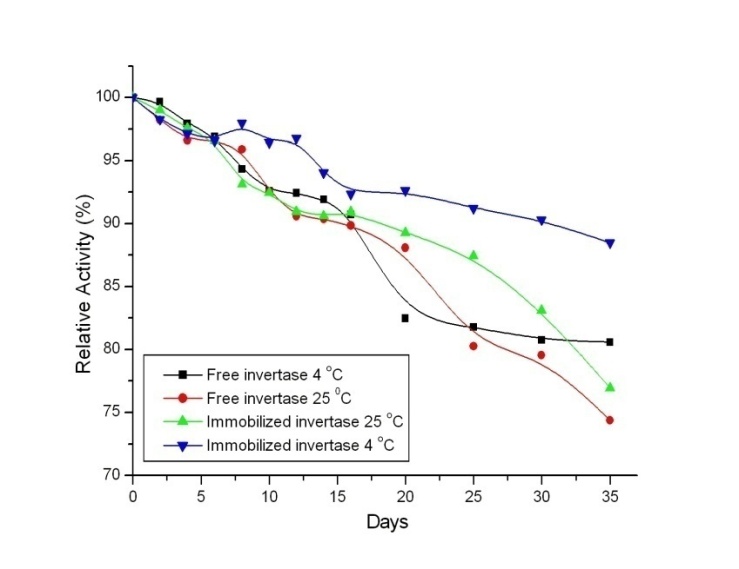 | Figure 5. Storage Stability of the free and immobilized invertase at 4 and 25˚C |
4.6. Effect of PH on the Adsorption
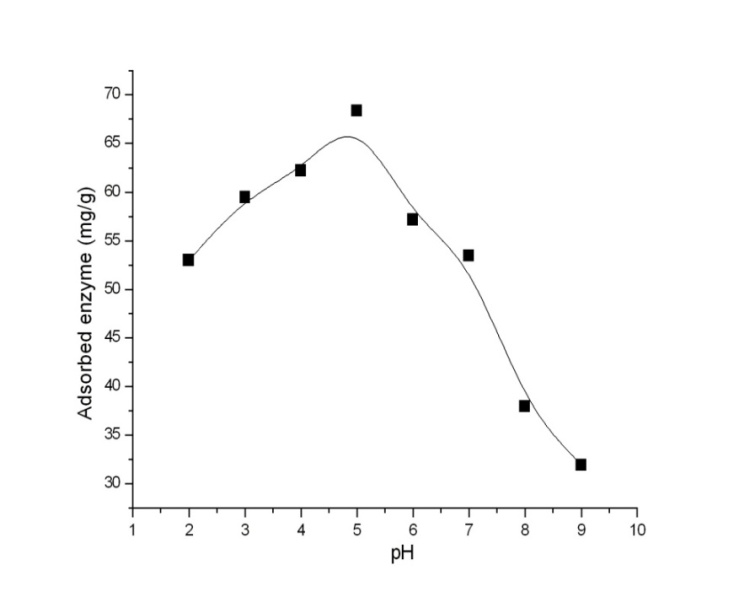 | Figure 6. Effect of pH on invertase adsorption onto of Cu2+ chelated functionalized Eupergit® C beads: invertase concentration, 0,8 mg/ml; time, 3 h; T, 20˚C |
4.7. Adsorption and Desorption Cycle
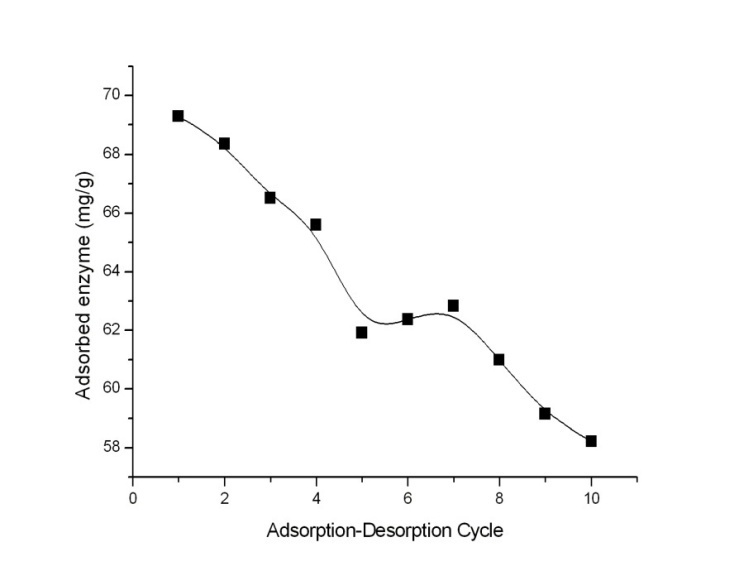 | Figure 7. Repeated use of immobilized invertase; invertase concentration 0,8 mg/ml; pH 5,0; time, 3 h; T, 20˚C |
5. Conclusions
- In this study, Eupergit® C beads were functionalized with aminotriazole groups for the reversible immobilization of enzymes. Metal-chelating ligand release is a common problem that is encountered in any immobilized metal-chelate affinity adsorption technique, which causes a decrease in the adsorption capacity. It is well known that metal-chelating ligand leakage from the adsorbent causes contaminations that will interfere with the analysis of the purified protein. Therefore, the metal- chelating ligand immobilization step was eliminated in this approach. The Michaelis-Menten kinetic constants Km and Vmax of the free and immobilized invertase were determined which revealed that the affinity of invertase to sucrose decreased after immobilization. The properties of the free and the immobilized invertase were compared and the results indicated that the stability of the immobilized invertase towards temperature, pH reusability, and storage was enhanced by immobilization. To conclude, the aminotriazole functionalized EC beads revealed good properties as adsorptive beads and will therefore prove to be useful for applications in biotechnology.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML