| [1] | Martindale, W. Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009 |
| [2] | United States Pharmacopoeia: USA 23 NF 18 Inc.; US Pharmacopoeia: USA, 1995 |
| [3] | British Pharmacopoeia; Her Majesty’s Stationary Office: London, UK, 1998 |
| [4] | S. S. M. Hassan, M. M. Abou-Sekkina, M. A. El-Ries, A. A. Wassel, “Polymeric matrix membrane sensors for sensitive potentiometric determination of some β-blockers in pharmaceutical preparations,” J. Pharm. Biomed. Anal. Vol. 32, pp. 175-180, 2003 |
| [5] | S. Khalil, M. M. El-Rabiehi, “Indirect atomic absorption spectrometric determination of pindolol, propranolol and levamisole hydrochlorides based on formation of ion associates with manganese thiocyanate and potassium ferricyanide,” J. Pharm. Biomed. Anal. vol. 22, pp. 7-12, 2000 |
| [6] | V. G. Dongrea, S. B. Shaha, P. P. Karmusea, M. Phadkeb, V.K. Jadhav. “Simultaneous determination of metoprolol succinate and amlodipine besylate in pharmaceutical dosage form by HPLC,” J. Pharm. Biomed. Anal .vol. 46, pp, 583-586, 2008 |
| [7] | A. K. Sarkar, D. Ghosh, A. Das, P. S. Selvan, K. V. Gowda, U. Mandal, A. Bose, S. Agarwal, U. Bhaumik, T.K. Pal, “Simultaneous determination of metoprolol succinate and amlodipine besylate in human plasma by liquid chromatography-tandem mass spectrometry method and its application in bioequivalence study,” J. Chromatogr. B vol. 873, pp. 77-85, 2008 |
| [8] | B. R. Simmons, J. T. Stewart, “HPLC separation of selected cardiovascular agents on underivatized silica using an aqueous organic mobile phase,” J. Liq. Chromatogr. Vol. 17, pp. 2675-2690, 1994 |
| [9] | P. Hubert, P. Chiap, M. Moors, B. Bourguignon, D. L. Massart, J. Crommen, “Knowledge-based system for the automated solid-phase extraction of basic drugs from plasma coupled with their liquid chromatographic determination: Application to the biodetermination of β-receptor blocking agents,” J. Chromatogr. A. vol. 665, pp. 87-99, 1994 |
| [10] | B. Mistry, J. Lesile, N. E. Eddington, “A sensitive assay of metoprolol and its major metabolite α-hydroxy metoprolol in human plasma and determination of dextromethorphan and its metabolite dextrorphan in urine with high performance liquid chromatography and fluorometric detection,” J. Pharm. Biomed. Anal. vol. 16, pp. 1041-1049, 1998 |
| [11] | V. L. Herring, T. L. Bastian, R. L. Lolende, “Solid-phase extraction and direct high-performance liquid chromatographic determination of metoprolol enantiomers in plasma,” J. Chromatogr. Vol. 567, pp. 221-227, 1991 |
| [12] | S. Uzu, K. Imai, K. Nakashima, S. Akiyama, “Use of 4-(N,N-dimethilaminosulphonyl)-7-fluoro-2,1,3-benzoxadiazole as a labelling reagent for peroxyoxalate chemiluminescence detection and its application to the determination of the ß-blocker metoprolol in serum by high-performance liquid chromatography,” Analyst vol.116, pp. 1353-1357, 1991 |
| [13] | R. J. Straka, K. A. Johnson, P. S. Marshall, R. P. Remmel, “Analysis of metoprolol enantiomers in human serum by liquid chromatography on a cellulose based chiral stationary phase,” J. Chromatogr. Vol. 530, pp. 83-93, 1990 |
| [14] | M. D. Phale, P. D. Hamrapurkar, “A validated and simplified RP-HPLC of metoprolol succinate from bulk drugs,” Asian J. Res. Chem. Vol. 119, pp. 119-122, 2009 |
| [15] | Y. Horai, T. Ishizaki, M. Kusaka, G. Tsujimoto, K. Hashimoto, “Simultaneous determination of metoprolol and[alpha]-hydroxymetoprolol in human plasma and urine by liquid chromatography with a preliminary observation on metoprolol oxidation in Japanese subjects,” Ther. Drug Monit. vol. 10, pp. 428-433, 1988 |
| [16] | M. I. Walash, A. M. El-Brashy, M. E. Metwally, A. Abdelal, “A spectrophotometric determination of penicillamine and carbocisteine based on formation of metal complexes,” Farmaco vol. 59 , pp. 493-503, 2004 |
| [17] | A. Asan, I. Isıldak, M. Andac, F. Yilmaz, “A simple and selective flow-injection spectrophotometric determination of copper(II) by using acetylsalicylhydroxamic acid,” Talanta vol. 60, pp. 861-866, 2003 |
| [18] | A. E. E. Ali, “Ternary complexes of copper(II) and their use in the spectrophotometric determination of copper in alloys and rocks,” Can. J. Chem. vol. 71, pp. 1002-1005, 1993 |
| [19] | G. Alberti, R. Biesuz, A. Profumo, M. Pesavento, “Determination of the total concentration and speciation of Al(III) in tea infusions,” J. Inorg. Biochem. vol.97, pp. 79-88, 2003 |
| [20] | A. Golcu, C. Yucesoy, S. Serin, “Spectrophotometric determination of some beta-blockers in dosage forms based on complex formation with Cu(II) and Co(II),” Farmaco vol. 59, pp. 487-492, 2004 |
| [21] | A. Golcu, M. Dolaz, S. Serin, “Spectrophotometric determination of propranolol as Cu(II), Ni(II) and Co(II) dithiocarbamate complexes,” Turk. J. Chem. vol. 25, pp. 485-490, 2001 |
| [22] | A. Golcu, Spectrophotometric determination of furosemide in pharmaceutical dosage forms using complex formation with Cu(II),” J. Anal. Chem. vol. 61, pp. 748-754, 2006 |
| [23] | R. Maccari, R. Ottana, B. Bottari, E, Rotondo, M. G. Vigorita, “In vitro advanced antimyco-bacterial screening of cobalt(II) and copper(II) complexes of fluorinated isonicotinoyl-hydrazones,” Bioorg. Med. Chem. Lett. Vol. 14, pp. 5731-5733, 2004 |
| [24] | P. R. Bontchev, B. B. Ivanova, D. R. Mehandjiev, D. S. Ivanov, “Complexation of Cu(II) with α-[(ethylamino) methyl]-3-hydroxy, benzenemethanol (Effortil). Crystal structure of the mononuclear Cu(II) complex with Effortil,” Polyhedron vol. 19, pp. 1843-1848, 2000 |
| [25] | S. Serin, “New vic-dioxime transition metal complexes,” Tran. Met. Chem. vol. 26, pp. 300-306, 2001 |
| [26] | K. J. Oberhausen, J. F. Richardson, R. M. Buchanan, J. K. McCusker, D. N. Hendrickson, J. M. Latour, “Synthesis and characterization of dinuclear copper(II) complexes of the dinucleating ligand 2,6-bis[(bis((1-methylimidazol-2-yl) methyl)amino)methyl]-4-methylphenol,” Inorg. Chem. vol. 30, pp. 1357-1365, 1991 |
| [27] | A. Golcu, M. Tumer, H. Demirelli, “Wheatley, R. A. Cd(II) and Cu(II) complexes of polydentate schiff base ligands: Synthesis, characterisation, properties and biological activity,” Inorg. Chim. Acta vol. 358, pp. 1785-1797, 2005 |
| [28] | M. Dolaz, M. Tumer, A. Golcu, S. Serin, “Synthesis and spectrophotometric investigation of a new vic-dioxime ligand and its transition metal complexes,” Turk. J. Chem. vol. 25, pp. 491-500, 2001 |
| [29] | J. B. Goodenough, “Magnetism and the Chemical Bond; Interscience (Wiley): New York, NY, USA, 1963 |
| [30] | A. Golcu, C. Yucesoy, S. Serin, “Synthesis, characterization of metal complexes of acebutolol, atenolol and propranolol-antihypertension drugs,” Synth. React. Inorg. Metal. vol. pp. 1259-1275, 2004 |
| [31] | H. Muslu, A. Golcu, S. A. Ozkan, “Electrochemical study of ceftazidime-copper(II) complex: Synthesis, characterization, biological activity and analytical application to pharmaceutical dosage forms,” Curr. Anal. Chem. vol. 6, pp. 299-309, 2010 |
| [32] | D. Tarinc, B. Dogan-Topal, M. Dolaz, A. Golcu, S. A. Ozkan, “Synthesis, characterization, biological activity and voltammetric behavior and determination of cefaclor metal complexes,” Curr. Anal. Chem. vol. 6, pp. 316- 328, 2010 |
| [33] | H. Miyano, T. Toyo’oka, K. Imai, “Further studies on the reaction of amines and proteins with 4-fluoro-7-nitrobenzo- 2-oxa-1,3-diazole,” Anal. Chim. Acta vol. 170, pp. 81-87, 1985 |
| [34] | Incezdy, J. Analytical Application of Complex Equilibria; Ellis Horwood Ltd.: England, UK, 1976 |
| [35] | A. Golcu, B. Dogan, S.A. Ozkan, “Anodic voltammetric behaviour and determination of cefixime in pharmaceutical dosage forms and biological fluids,” Talanta vol.67, pp. 703-712, 2005 |


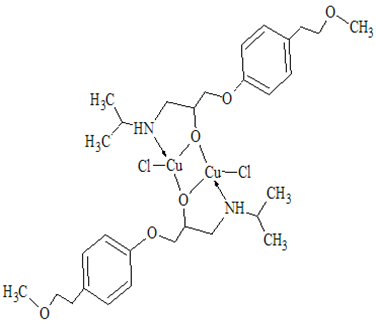
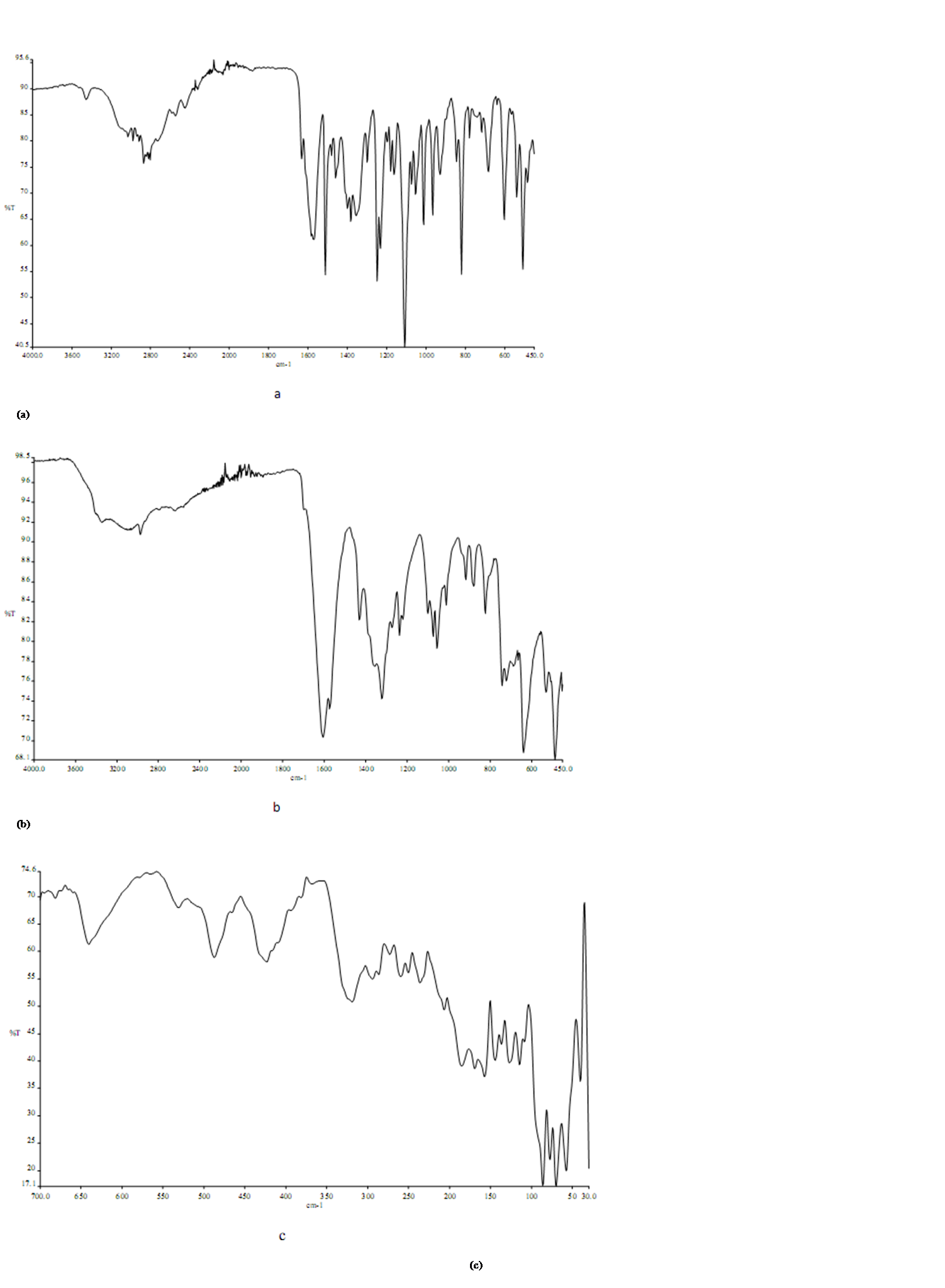
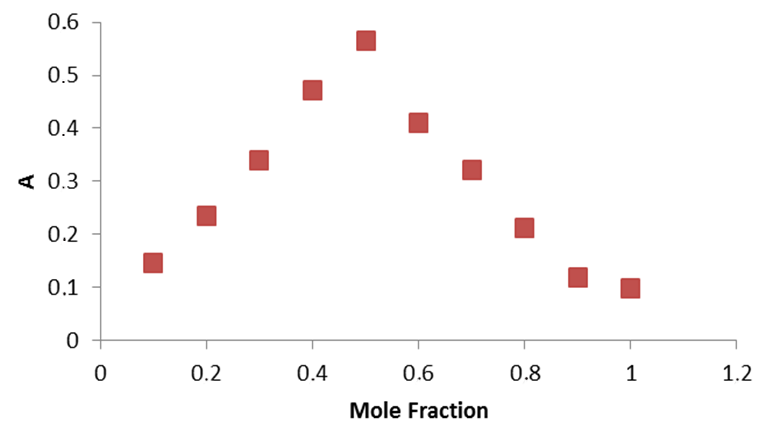
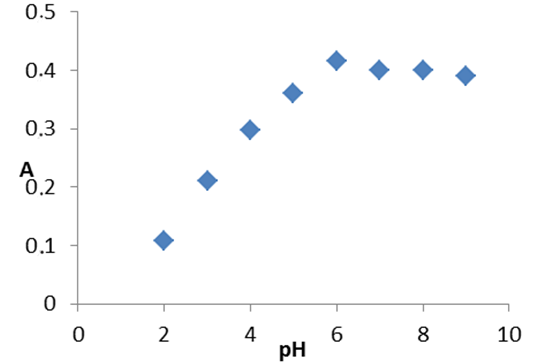
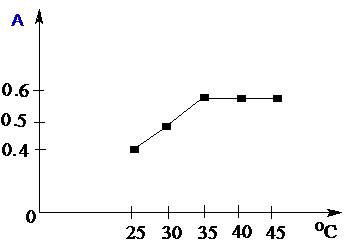
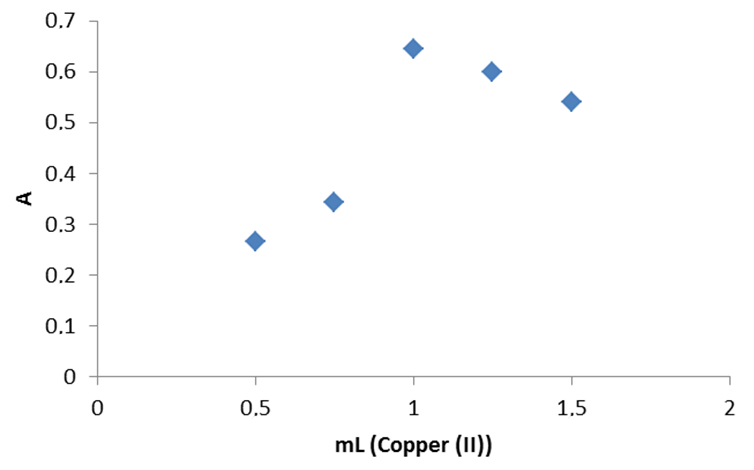
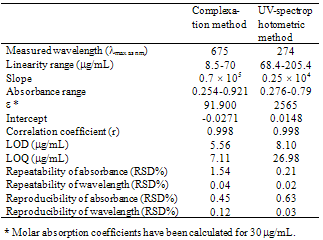
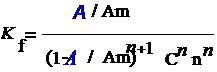 where A is maximum absorbance, Am is the absorbance corresponding to intersection of the two tangests of the curve in Figure 3, C is the concentration corresponding to maximum absorbance, n is the amount of the drug in reaction product. Using this equation, Kf was found to be equal to 1.5 × 1010. Under the described experimental conditions, the relation between the absorbance at 675 nm with the concentration of MPT was linear over the 8.5-70 μg/mL range. Linear regression analysis of the concentration-absorption data gave the following equation:A = 70,000C − 0.00271, r = 0.998where C is the concentration in μg/mL, A is the absorbance and ‘r’ is the correlation coefficient showing excellent linearity. The limit of detection (LOD), expressed as the concentration at a signal-to-noise ratio 3 s/m, was 5.56 μg/mL and the limit of quantification (LOQ), expressed as the concentration at a signal-to-noise ratio 10 s/m, 7.11 μg/mL[31,32,35].
where A is maximum absorbance, Am is the absorbance corresponding to intersection of the two tangests of the curve in Figure 3, C is the concentration corresponding to maximum absorbance, n is the amount of the drug in reaction product. Using this equation, Kf was found to be equal to 1.5 × 1010. Under the described experimental conditions, the relation between the absorbance at 675 nm with the concentration of MPT was linear over the 8.5-70 μg/mL range. Linear regression analysis of the concentration-absorption data gave the following equation:A = 70,000C − 0.00271, r = 0.998where C is the concentration in μg/mL, A is the absorbance and ‘r’ is the correlation coefficient showing excellent linearity. The limit of detection (LOD), expressed as the concentration at a signal-to-noise ratio 3 s/m, was 5.56 μg/mL and the limit of quantification (LOQ), expressed as the concentration at a signal-to-noise ratio 10 s/m, 7.11 μg/mL[31,32,35]. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML