-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2020; 10(1): 1-8
doi:10.5923/j.biophysics.20201001.01

Analytical Approach to Cancer Therapy by Inhibition of Blockade Regulation in the Context of Protein Vibration
Brajagopal Majumder
Retired Reader, Department of Physics, Government Degree College, Agartala, Tripura, India
Correspondence to: Brajagopal Majumder, Retired Reader, Department of Physics, Government Degree College, Agartala, Tripura, India.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A brief account of discovery of cancer therapy by overcoming the negative immune regulation of cancerous antigens (proteins) with cloned antibodies (proteins) for which Nobel Prize was awarded in Physiology or Medicine in 2018 has been analytically discussed here. These antibodies are now regarded as checkpoint inhibitors. These discoveries have opened a new channel for better treatment of different types of cancer. In view of these successful endeavours, attempts have been made by the author to explain the functioning of both curative and immune role of certain antibodies (proteins) like MDX 010, Nivolum ab and pembrolizum ab against cancer causing antigens like CTLA 4, PD 1, PDL 1, PDL 2, etc., in the context of Protein vibration. A short note on the role of protein vibration was also discussed.
Keywords: Immune responses, Blockades, Cancerous antigens, Cloned antibodies, T cell receptors, Co-stimulation, Inhibitors
Cite this paper: Brajagopal Majumder, Analytical Approach to Cancer Therapy by Inhibition of Blockade Regulation in the Context of Protein Vibration, International Journal of Biophysics , Vol. 10 No. 1, 2020, pp. 1-8. doi: 10.5923/j.biophysics.20201001.01.
Article Outline
1. Introduction
- It is held by Hanahan and Weinberg [1] that all cancers display certain hallmarks, though etiologic and pathogenesis of cancer are found complex. It is now established that each hallmark being influenced by multiple pathway is found to render dysfunction by alteration in the genes of the cancer cells. While some of these genetic changes are inherited via the germ line DNA, majority is found to be acquired as mutations in somatic cells due to intervention of a number of external factors.It is reflected in the report of Global Cancer Observatory, 2018 [2] managed by WHO that millions of population (more than 18 million) are estimated to be affected with cancer in 2018. This constitutes the fact that one in three is developed with cancer in economically developed countries and it is also estimated that this will turn to one in two in next two decades. The scenario of the diseases though devastating, yet life of the patients can be prolonged by different treatments which are usually within the economic capability of the patients. But the reverse is found in countries with less developed economy which results in fewer cures and reduced amount of survival of the patients. Thus treatment of cancer patients is found critical from the view point of application of appropriate medicine. These are also costly enough. In spite of all these hurdles, scientists of the day are toiling their best to innovate multiple ways of treatment of the patients with cost effective approach too.A number of discoveries have magnified the present knowledge about cause and remedies of cancer. Most of these were awarded Nobel Prizes in Physiology or Medicine. These include(1) Identification of infection as an etiological factor by Rous in 1966.(2) Discovery of human papilloma virus as a cause of cervical cancer by zur Hauzen in 2008. (3) Establishing the relation between cellular and viral genes in pathogenesis like (a) integration of retroviral genetic information into DNA by Batimore, Dulbecco and Temin in 1975 and (b) the cellular origin of viral oncogenes in 1989. Moreover, a good number of novel therapies discovered by a good number of scientists were also awarded Nobel Prizes in Physiology or Medicine. Some of these include(1) Hormone treatment for prostate cancer by Huggins (1966).(2) Discovery of the mechanism of cytostatic drugs affecting the metabolism of nucleic acid by Elion and Hitchins (1988).(3) Discovery of bone marrow transplantation for treating blood cancer by Thomas (1990).These therapies are being used as complement to the traditional methods of surgery and radiotherapy since latter part of the 20th century.In addition, U.S. Food and Drug Administration (FDA) has approved (2a) good number (more than a dozen) of monoclonal antibodies (mAbs) for treatment of cancer. These are being used for both purposes of cancer treatment as curative agent and also an immune therapy agent. But identification and uses of these antibodies are not so easy. The antibodies so far cloned are now classified as naked monoclonal antibody and conjugated monoclonal antibody.Naked antibodies work by themselves. No drug or radioactive material is attached to these. These are the most common type of mAbs for treating cancer. Some of these boost antigens found in lymphocyte (included in leukemia) cells, attracts immune cells to destroy these cells. Other naked mAbs like trastuzum ab (Herceptin) works mainly by attaching and blocking antigen like HER2 protein responsible for breast and stomach cancer cells.Monoclonal antibodies (mAbs) joined to chemotherapy drug or radioactive material are called conjugated antibodies. These can be classified as radiolabeled and chemolabeled antibodies. As for example, Ibritumomab tiuxetan (Zevalin) a radioactive antibody works against CD20 antigen which is found in lymphocytes generally known as B cells. On the other hand, chemolabaled antibodies or antibody-drug conjugates (ADCs) are attached to chemotherapy. These are of two types as Brentuximab vedotin (Adcetris) and Ado-trastuzumab emtansine (called TDM 1). While Brentuximab together targets CD 30 antigen by attaching with chemo drug (MMAE) used to treat Hodgkin lymphoma and anaplastic large cell lymphoma. Ado-trastuzumab entansine (Kadcyla) antibody targets the HER2 protein attached to chemo drug called DMI is used to treat breast cancer, etc.
2. Background of Immune Therapy to Cancer
- In addition to successful chemotherapy endeavours, scholars have been trying their best to involve them to the principle of immune therapy. The basic understanding of the immune system revealed in the beginning of the 20th Century when Leo Loeb discussed the possible role of immunity for the growth of experimentally transplanted tumours [3] with establishing inbred mouse strains. Using this possibility, it was observed by Snell and Higgins in 1951 that "alleles within the histocompatibility-2 (H-2) locus in the mouse were the key determinants for tumours transplantation". George D. Snell was awarded Nobel Prize for his discovery in 1980. These discoveries paved the way for further understanding; the phenomenon of discriminating self from 'non-self' with respect to using lymphocyte molecules of the Major Histo Compatibility Complex (MHC). Based on this Peter C. Doherty and Rolf M. Zinkernagel discovered "the specificity of the cell mediated immune defence" for which they were awarded Nobel Prize in 1996. Above these scientists, Paul Ehrlich shared the prize with llya llyich Mechnikov in 1908 for reorganization of his in-depth study in the potential effects of natural and acquired immunity for cancer [4]. Sir Frank Macfariane Burnet shared Nobel Prize in 1960 for his discovery of acquired immunological tolerance which proposed that "immune system serves as a surveillance system for cancer" [5].Moreover, a number of fundamental discoveries in tumour immunology like demonstration of tumour-specific antigens by Klein and Klein in 1962 and their molecular nature by Lurquin et al. in 1989, cytotoxic Tcell killing of tumour cells by Brunet et al. in 1968, tumour-infiltrating lymphocytes by Klein et al. in 1977 [6,7,8,9] and their role in immunotherapy by Rosenberg et al. in 1986 [10], immune selection and immune editing during tumour progression [11], etc., observed profound and beneficial effects on tumour growth with respect to animal.
3. T Cell Activition
- Development of monoclonal antibody technology in 1980 by George J. F. Kohler and Cesar Milstein for which they were awarded Novel Prize in 1984 opened the door for identification of novel cell surface makers though prior to this, antigen-specific receptor on B cell had already been characterized. Thus, it may be held that understanding of cellular adaptive immune responses was started to take place from 1980. During this decade, T cell receptor (TCR) was identified and its interaction with MHC-associated peptides on antigen presenting cells was revealed. This paved the way for cloning a gene, namely, interleukin-2, an important regulator of T cells. This results in understanding the principle of signaling of TCR. As a result, CD 28 was identified as the first cell surface molecule in TCR co-stimulation. This was recognized as a monoclonal antibody protein/protein receptor on T cells and thymocytes [12]. Since then rapid development in this field occurred with identification of B7 molecule as legend for CD 28. B 7 is now known as CD 80 and is found to be expressed on antigen-presenting cells by Lindsten et al. in 1993 [13]. It was also observed that like CD 28, CTLA 4 binds to B 7. Prior to 1993, the function of CTLA 4 was unknown but both CD 28 and CTLA 4 belong to the immunoglobulin super family. It is now established that CTLA 4 resides intracellularly in resting T cells but translocates rapidly to the membrane after activation [14,15]. In 2000, it was also observed by the scholar [16] that CTLA 4 is expressed as a membrane protein in regulatory T cells [17] and acted in similar co-stimulatory way as that of CD 28. However, different group of scholars by Krummel and Allison in 1995 [18] and the group of Allison with Hurwitz and others [19] came to the conclusion that CTLA 4 serves as a negative regulator of T cell activation, which means CTLA 4 induces negative signaling against positive signals elicited by TCR and co-stimulatory receptors.
4. Role of Immune Checkpoint Inhibitors
- The aforementioned findings opened the door to the scientists to develop treatments for autoimmunity. Among these scientists, James P. Allison attempted to find a cure for cancer by blocking the negative effects that are induced by CTLA 4. By the end of 1994, Allison set up the first experiment in his laboratory at the University of California, Berkley to treat tumour transplanted mice with monoclonal antibodies against CTLA 4. The Allison laboratory carried this experiment with number of cancer affected animals other than mice. These include tumour models including prostate cancer [20], mammary tumours [21] and melanoma [22]. Some of these experiments were carried with combined therapies of anti-CTLA 4 and immune stimulatory cytokine like GM-CSF (granulocyte-macrophage colony stimulating factor). Thus, it is observed that monoclonal antibodies are capable to unleash responses against tumours and this is referred to as Immune Checkpoint Inhibitors (ICIs).Allison had to face trouble for translating his research into clinical program, since no pharmaceutical company showed least interest in this respect. At last a small biotech namely Medarex came forward and ultimately an anti-CTLA 4 igG1, monoclonal antibody named MDX010 and popularly known as ipilimumab was developed. Nine melanoma patients [23] were administered MDX010 in phase-1 clinical trial with a single dose of 3 mg/kg and the response was recorded. Complete regression was reported in another trial in some treated melanoma patients. A special feature like increase in tumour volume, namely "pseudoprogression" owing to infiltration of immune cells rather than reducing it was also observed. Major breakthrough was observed in phase III trial when metastatic melanoma showed significant increase in survival [24]. Based on this trial, FDA and EMA approved the treatment of anti CTLA-4.In 2018, Nobel Prize was shared by Tasuku Honjo of Kyoto University in Japan for almost similar discovery of James P. Allison. PD-1 (CD 279) was first identified and cloned by Tasuku Honjo's group at Kyoto University in 1990 [25] prior to Allison's discovery of CTLA 4 inhibition for treating cancer. Two studies, one from Chen's laboratory [26] and the other from the Kyoto groups of Minato and Honjo [27] focused on PDL 1 molecules are found to be expressed on tumour cells and it was held after demonstration that these expressions would "protect transformed cells from immune attack in vivo and this could be reversed by suitable antibodies to PDL 1". Hence, it becomes necessary to devise the path of using appropriate antibody which may be involved in immune response to tumour.The 1st experiment with antibody to the receptor PD 1 responsible for cancer conducted with mice was published in 2005 by Honjo's groups. The paper presented several pathways with respect to anti PD-1 treatment like '(i) induction of immune responses also to tumours which do not express detectable PD 1 or PDL 2; (ii) it is at least as efficient as CTLA 4 therapy to tumours; (iii) it shows less severe auto immune side effects than CTLA 4' [28]. The name of the antibody as synthesized is nivolumab and it was also observed that the drug was well tolerated in its 1st phase trial during 2006 [29]. Two years later it was reported [30] that the antibody used in 296 patients in phase 1 trial indicated remarkable efficacy in treatment of different types of tumours which were found in advanced stage. After a good number of trials during the last 15 years with anti-CTLA 4 antibodies and 12 years of the same with anti-PD 1 antibodies for treatment of different types of cancer, it was held that anti-PD 1 antibodies treatment yields better response as compared to that of anti-CTLA 4 antibody. However, combined therapy with respect to ICIs against PD 1 or its legend PDL 1 and CTLA 4 is found to respond "stronger anti-tumour effects" in melanoma [31] and renal cell carcinoma [32]. Now the question arises, what would be the functioning mechanism of primary as well as acquired resistance of tumour cells to anti-CTLA 4 and anti-PD 1 therapy. Though there are number of interesting observations regarding up-streaming of T cell activation like (i) mutational load in the tumour expressed with antigens, (ii) previous infections or vaccination, (iii) micro environment and microbiome in niches [33,34], the actual mechanism where antibodies interfere with negative responses are full of complexes. Allison's group provided four possible evidences for four different mechanisms like (i) "blockade of a direct inhibitory signaling pathway in effectors T cells, (ii) sequestration of activating legends on antigen presenting cells, (iii) interference with the function of regulatory T cells, and (iv) elimination of regulatory T cells".All these findings follow biochemical processes. The characteristics of these biochemical processes require to be analysed from the view point of physical phenomenon too. In a recent paper, the author [35] explained analytically the modulating effects of Heat Shock Proteins (HSPs) towards immune responses of cancer in the context of protein vibration. Heat Shock proteins are found to be either auto induced or to be induced due to external administration of therapeutic agents including some kinds of Indian Medicinal Herbs like Becopa Monnieri (BM), Ashwagandha [36,37] etc. These proteins are found to modulate the immune system by stimulating both inmate and adaptive responses. The paper [35] focuses on the dynamism of modulating effects of HSPs towards immune responses of cancerous proteins based on the vibration frequencies of both cancer causing proteins or protein receptors and cancer curing proteins (Say HSPs).
5. Proposed Analytical Approach
- In view of the aforementioned discussion, the author attempts to explain the functioning of antibody (cancer curing protein) with respect to cancerous antigens from the view point of protein vibration. It is to be noted that protein vibrations enable proteins to change their shape quickly so that they can readily bind to other proteins and also now held responsible for conformational changes with respect to protein misfolding which is being considered as causes of a number of disorders in human bodies. This constitutes the basis of the present study. The author has been working analytically on the utility of protein vibration in learning and memory losses along with therapeutic aspects of old age diseases like Parkinson, Alzheimer, Huntington, [38] etc. The analytical results evaluated on the basis of protein vibration hypothesis are found to be compatible with those derived from experimental results of disaggregation of aggregated protein responsible for old age diseases in vitro phase which thus established the rule of protein vibration in mammalian body.Now it is an established fact that proteins vibrate in vivo either due to inherent electrostatic force (potential) of the order of 0.8 to 30 mv with respect to variable Ph by Sivasankar et al. in1998 [39] or due to external stimuli. In a paper [40], the author suggested that the vibration characteristics of protein depend on the magnitudes of molecular weights of the concerned protein. It was also held by the authors that the more the molecular weight of protein, the less is the number of vibration frequency. On the other hand, the less value of molecular weight of protein is, the more the number of frequency. Thus the vibration pattern of protein may be described as shown in Figure 1.
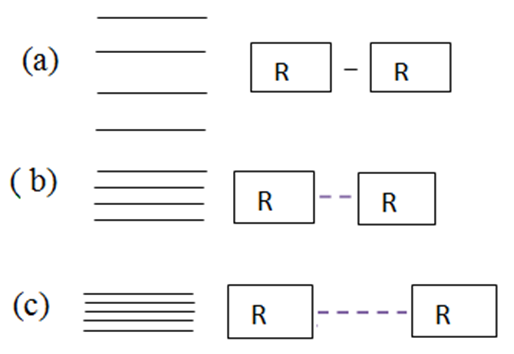 | Figure 1. Vibration energy spacing of protein molecules |
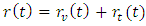 where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation:
where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation: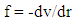 Which means force is nothing but rate of change of potential:
Which means force is nothing but rate of change of potential: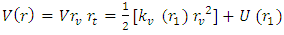 | (1) |
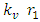 is the vibration component of the force and U (r) stands for the translational component of the same. Since the translational component of the force is very small with respect to the vibration component of the same, the second term of Equation 1 may be neglected. So Equation 1 may be rewritten as:
is the vibration component of the force and U (r) stands for the translational component of the same. Since the translational component of the force is very small with respect to the vibration component of the same, the second term of Equation 1 may be neglected. So Equation 1 may be rewritten as: 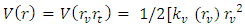 | (2) |
 | Figure 2. Oscillation of vibrating particle |
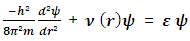 | (3) |
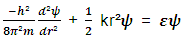 | (4) |
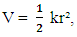 while other symbols stand for usual meaning.The energy of the allowed vibration states derived from the solutions of the Schrodinger’s Equation is
while other symbols stand for usual meaning.The energy of the allowed vibration states derived from the solutions of the Schrodinger’s Equation is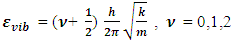 | (5) |
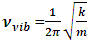 | (6) |
6. Results and Discussion
- Physical characteristics of the concerned cancer causing antigens (proteins / receptor proteins) with respective cancer curing antibiotics (proteins) are shown in Table 1.Based on Equations 6, let us evaluate the vibration frequencies of the aforementioned noted proteins shown in Table 2. It is revealed from Table 2 that the numerical values of vibration frequencies of CTLA 4, PD 1, and PDL 1 as some of cancer causing proteins (antigens) are almost of the same magnitudes. Since the molecular weights of CTLA 4, PD 1, and PDL 1 are almost in the same magnitudes, the vibration frequencies of all these proteins are also in same range. These are some of the physical characteristics of proteins and protein receptors responsible for causing cancers and tumors as considered from the view of protein vibration.Now let us pay attention to the role of antibodies like MDX-010, targeting CTLA 1, Nivolumab targeting PD 1 and pembrolizumab targeting PD 1 and PDL 1, the molecular weights of which are almost in the same range. From Tables 1 and 2, it is also found that the vibration frequencies of these antibodies (Proteins) are also in same range.
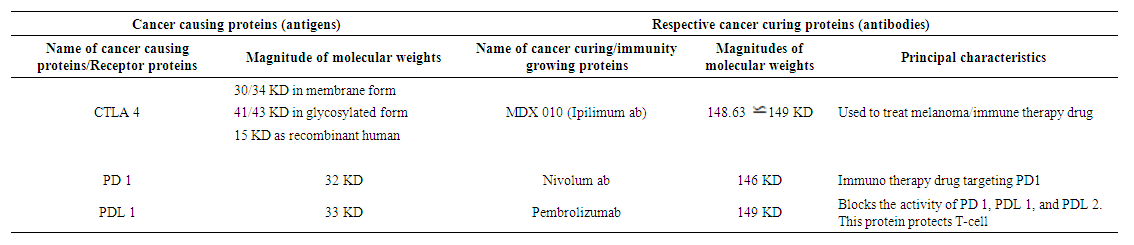 | Table 1. Characteristics of cancer causing antigen and cancer curing antibodies |
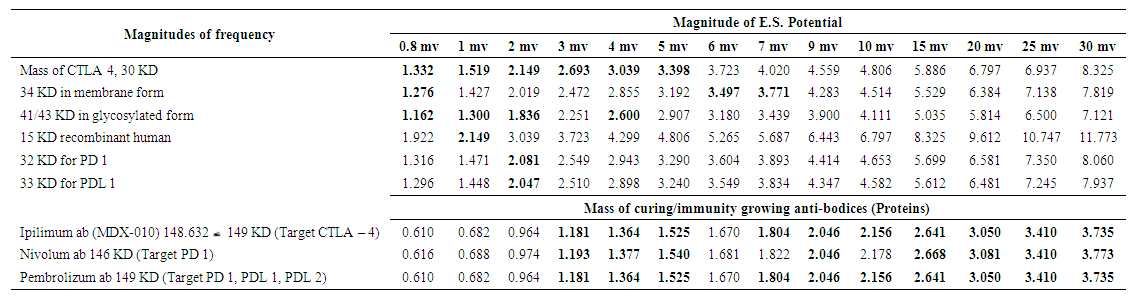 | Table 2. Frequencies (m-1) generated by different proteins in the presence of ES potential (0.8 to 30 mv) |
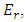 the energy value in r level may contribute to
the energy value in r level may contribute to 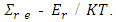 The term
The term 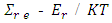 [41] is known as partition function. But when loose binding of the molecules or separation of chains occurs naturally or by any means, the levels will be closer and several terms may remain present making the partition function greater and F the Gibb’s free energy per mol smaller (Majumder, 1997). The diagrammatical representation of these two cases is similar to those described in Figure 1.From Table 2, it reveals that vibration frequencies generated due to antigen proteins of masses 30, 32, 33, 34, and 41 KDs are found to be in parity levels of antibody proteins of masses m=146 and 149 KDs at certain levels of electrostatic potentials in between 0.8 and 6 mv. This means there would be strong binding of cancer curing antibodies to cancer causing antigens. These are shown in Table 2 by colour circles. In case of other values of electrostatic potential, the frequencies generated due to the same antigens and antibodies are not found to be in good unison. Rather they are found to be distributed in scatter forms. However, numerical values of frequencies generated due to antibody proteins for electrostatic potential of magnitudes 7 to 30 mv as found shown in Table 2 are lower than those of antigens for the same order of electrostatic potential. But the wavelength of the frequencies generated due to antibody proteins are higher than those generated by antigen proteins. These higher values of wavelength will be able to modulate the lower values of wavelength as it is found in frequency modulation of electromagnetic waves. Similarly, modulation of antibody protein will stand as change in signaling pathway of frequencies generated by cancer causing proteins.Practically speaking, vibration of proteins is nothing but vibration of electrons in hydrogen molecules which take part in binding of proteins. Hence, frequency generated due to protein vibration obviously takes part in binding process of proteins. In this context, both binding and modulation can be thought to be synonymous with frequency modulation in electromagnetic propagation process which is nothing but change in signaling pathway. Recently, the author (Majumder, 2017) explained analytically the modulating effects of Heat Shock Proteins (HSP) towards immune responses of cancer from the view point of protein vibration. But most of the interventions of the therapeutic uses of HSP are still in experimental/trial stage with mice, along with demonstration on vaccination with HSP 70 derived from Math A sarcoma in mice. On the other hand, therapeutic uses in the form of injection of antibodies (proteins like MDX010, Nivolumam, pembrolizumab) against cancerous antigen (proteins) like CTLA 4, PD 1, PDL 1, PDL 2, etc., are significantly increasing. And hence the possibility of getting cure from cancerous antigens with injection of antibodies as mentioned is now found stable particularly with comparison to modulating effects of Heat Shock Proteins towards immune responses of cancer since induction of positive Heat Shock Proteins in body system of mammalian bodies is uncertain. Thus treating cancerous antigens like CTLA 4, PD 1, PDL 1, andPDL 2 with injection of antibodies like MDX010 and others for which Noble Prizes in Physiology or Medicine has been awarded for 2018, will stand certainly in good stead for mankind.
[41] is known as partition function. But when loose binding of the molecules or separation of chains occurs naturally or by any means, the levels will be closer and several terms may remain present making the partition function greater and F the Gibb’s free energy per mol smaller (Majumder, 1997). The diagrammatical representation of these two cases is similar to those described in Figure 1.From Table 2, it reveals that vibration frequencies generated due to antigen proteins of masses 30, 32, 33, 34, and 41 KDs are found to be in parity levels of antibody proteins of masses m=146 and 149 KDs at certain levels of electrostatic potentials in between 0.8 and 6 mv. This means there would be strong binding of cancer curing antibodies to cancer causing antigens. These are shown in Table 2 by colour circles. In case of other values of electrostatic potential, the frequencies generated due to the same antigens and antibodies are not found to be in good unison. Rather they are found to be distributed in scatter forms. However, numerical values of frequencies generated due to antibody proteins for electrostatic potential of magnitudes 7 to 30 mv as found shown in Table 2 are lower than those of antigens for the same order of electrostatic potential. But the wavelength of the frequencies generated due to antibody proteins are higher than those generated by antigen proteins. These higher values of wavelength will be able to modulate the lower values of wavelength as it is found in frequency modulation of electromagnetic waves. Similarly, modulation of antibody protein will stand as change in signaling pathway of frequencies generated by cancer causing proteins.Practically speaking, vibration of proteins is nothing but vibration of electrons in hydrogen molecules which take part in binding of proteins. Hence, frequency generated due to protein vibration obviously takes part in binding process of proteins. In this context, both binding and modulation can be thought to be synonymous with frequency modulation in electromagnetic propagation process which is nothing but change in signaling pathway. Recently, the author (Majumder, 2017) explained analytically the modulating effects of Heat Shock Proteins (HSP) towards immune responses of cancer from the view point of protein vibration. But most of the interventions of the therapeutic uses of HSP are still in experimental/trial stage with mice, along with demonstration on vaccination with HSP 70 derived from Math A sarcoma in mice. On the other hand, therapeutic uses in the form of injection of antibodies (proteins like MDX010, Nivolumam, pembrolizumab) against cancerous antigen (proteins) like CTLA 4, PD 1, PDL 1, PDL 2, etc., are significantly increasing. And hence the possibility of getting cure from cancerous antigens with injection of antibodies as mentioned is now found stable particularly with comparison to modulating effects of Heat Shock Proteins towards immune responses of cancer since induction of positive Heat Shock Proteins in body system of mammalian bodies is uncertain. Thus treating cancerous antigens like CTLA 4, PD 1, PDL 1, andPDL 2 with injection of antibodies like MDX010 and others for which Noble Prizes in Physiology or Medicine has been awarded for 2018, will stand certainly in good stead for mankind. 7. Conclusions
- The antibodies approved by US Food and Drug Administration are being used for long now. But the implications of cloned MDX-010 targeting CTLA 4 and other antibiotics like Nivolumab and Pembrolizumab targeting PD 1 and PDL 1, respectively or combination of both has nowadays been found to be more successful than those of other antibodies noted so far. Thus, the therapeutic uses of antibodies (proteins like MDX010, Nivolumab, Pembrolizumab etc.) against cancerous antigens like CTLA 4, PD 1, PDL 1, PDL 2, etc., are significantly increasing. This results in possibility of getting cure as well as modulating effects of immune responses on the part of the patients. These are found to be more effective in comparison with other endeavours undertaken by different group of scientists with other proteins like HSPs, etc. Recent interventions of therapeutic use of HSPs along with demonstration on vaccination with HSP 70 derived from Math A sarcoma in mice are still in trail stage. Hence, successful therapeutic use of cancer curing antibodies against respective antigen as discussed for which Nobel Prize was awarded in Medicine or Physiology in 2018 conferred great benefit on mankind by opening a new channel to almost stable cancer treatment.
ACKNOWLEDGEMENTS
- The author is thankful to Dr. U. C. De, associate professor, Chemistry Department, Tripura University, Agartala, Tripura, India and also thankful.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML