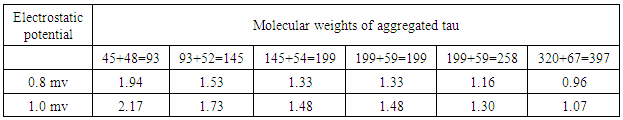-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2018; 8(1): 1-8
doi:10.5923/j.biophysics.20180801.01

Activation of Heat Shock Protein Induced by Curcumin to Prevent Huntington Disease- an Analytical Approach in the Context of Protein Vibration
Brajagopal Majumder
Retired Reader in Physics, Govt. Degree College, Agartala, Tripura, India
Correspondence to: Brajagopal Majumder, Retired Reader in Physics, Govt. Degree College, Agartala, Tripura, India.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Depending on aggregate size, amino acid sequence and degree of misfolding, protein aggregates are disaggregated by Heat Shock Proteins (HSPs). Natural products can induce SHPs. Curcumin- the chemical constituent of turmeric paste/powder is now being considered as the inducer of HSPs which are able to reduce aggregates of glutamine amino acids and can also modulate the signaling pathway of small fragments of glutamine molecules. The molecular mechanism of disaggregation of aggregated glutamines has been considered from the view point of protein vibration which is expected to be happened either due to inherent electrostatic potential of the cell or due to external stimuli. Here we have discussed the current knowledge on the role of HSP induced by curcumin particularly with respect to Huntington diseases (HD) in the context of Protein Vibration Approach.
Keywords: Curcumin, Heat Shock Proteins, Protein misfolding, Neurodegenerative diseases, Molecular Chaperones, Huntingtin protein (HTT), Huntington Disease
Cite this paper: Brajagopal Majumder, Activation of Heat Shock Protein Induced by Curcumin to Prevent Huntington Disease- an Analytical Approach in the Context of Protein Vibration, International Journal of Biophysics , Vol. 8 No. 1, 2018, pp. 1-8. doi: 10.5923/j.biophysics.20180801.01.
Article Outline
1. Introduction
- Accumulation of misfolded and aggregated amyloid proteins in intra and extracellular system is a common feature in a number of neurodegenerative diseases. Most of these diseases are silent killer. These progress slowly and persist rest of life [1]. The common features of these devastating diseases constitute synaptic loss, neurobehavioral abnormalities, impairment of learning and memory. The present knowledge supports the idea that these diseases might start at early stage of life but manifest at later stage especially during aging [2]. One of the most common of these diseases is Alzheimer’s diseases (AD) where amyloid beta protein (Aβ) is deposited in extracellular space and microtubule stabilizing protein tau as neurofibrillary tangle (NET) is deposited in intracellular space. Similarly, a number of proteins like alpha synuclein, huntingtin (HTT), prion are deposited in Parkinson’s (PD), Huntington’s (HD) and prion diseases respectively. In most of these devastating diseases the concerned amyloid proteins undergo conformational changes and become misfolded. These are deposited as insoluble proteinaceous aggregate inside or outside of cells. Therefore, adequate treatment requires to be started before onset of these diseases, failing which it would be hard to prevent later downstream of toxicity of these proteins. Several attempts have been undertaken to clear of these protein aggregates but no permanent solution has yet been emerged out. Recently, activation or expression of special kind of protein known as Heat Shock Protein (HSP) draws the attention of the scholar towards therapeutic strategies for removing these abnormal protein aggregates. Disaggregation of these abnormal protein aggregates has been discussed here from the view point of protein vibration –which is briefed in the methodology chapter.
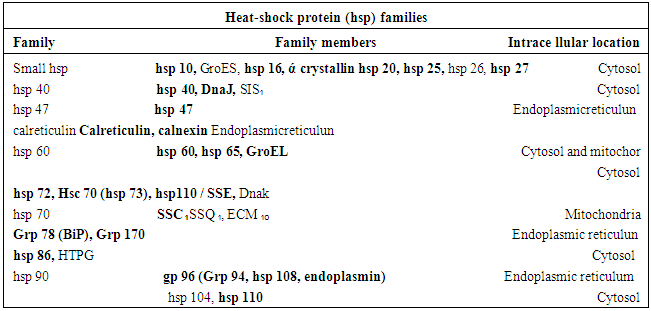
2. Characterization of Heat Shock Proteins and Their Functions
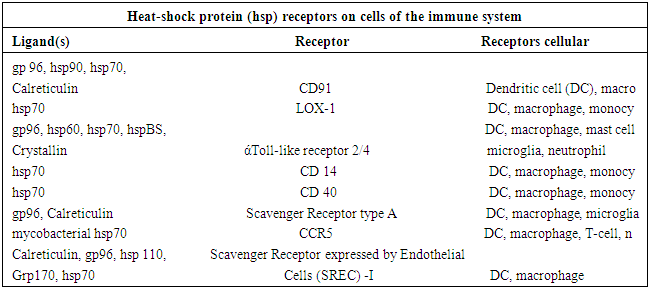
- Heat Shock Proteins (HSPs) are a class of proteins that are produced by cells in response to stressful conditions. They were first identified with respect to heat shock but are now found to be expressed during other stresses like exposure to cold [3], UV Light [4] and during wound healing or tissue remodeling [5]. Many members of this group perform chaperone function to refold misfolded proteins. HSPs are thus found to act as(i) Upregulators in stress and also as(ii) ChaperonesHSPs are found virtually in all living organism from bacteria to human. Synthesis of increased amount of proteins in Drosophila cells following stresses were first reported in 1974 [6]. Several HSPs now function as intracellular chaperones for other proteins. They play an important role in protein – protein interaction which aims to establish proper protein conformation and prevention of unwanted protein aggregation. Some HSPs are found to play a significant cardiovascular role [7]. On the other hand, extracellular and membrane bound HSPs are involved in binding antigens and presenting them to the immune system [8]. Thus a good number of HSPs have now been identified. They are usually named by their molecular weights and their family members are now categorized [9] asMultiple receptors present in dendrite and other cells bind to HSPs to modulate the immune system in vivo. Protein receptors which are found to play a role in binding with HSPs are tabled [9] asDistinct HSPs are found to posses the abilities of inducing cross – presentation of antigens. For example, it may be mentioned that four major chaperone proteins HSP – 70, HSP – 90, Grp – 94 and gp – 96 and calreticulin were identified in an extract prepared from human melanoma lines. These are functional in enhancing presentation of exogenous peptides. However, superior activity is observed in HSP-70 rich preparation that can be used in immune therapy of tumors and vaccine development [10].Now it is an established fact that most of the neurodegenerative diseases are due to abnormal protein aggregation which basically causes misfolding of the native protein with conformational changes. For example, alpha synuclein, tau isomers, huntingtin (HTT), prion proteins etc are found to be deposited in case of P.D, A.D, HD and prion diseases respectively. On the other hand, induction or expression of HSPs draws a special attention in removing the abnormal aggregates.The HSPs are highly associated with cellular defense mechanism. Now – a-days, it is found to regulate various cellular functions including protein folding, refolding of partially denatured proteins, protein transport across membrane, cytosheletal organization and apoptosis etc [11]. It acts as cytoprotective factor against deleterious environment stresses. Its activation includes also a wide range of client substrates including hormone receptor and also kinases, oncogenic proteins [12]. Different HSPs are localized in synapses and axons. These are found to regulate disposal of toxic aggregation like amyloid plaque and NFT in A.D, alpha synuclein in P.D, huntingtin (HTT) in H.D [13] and prion protein in Cretzfeldt – Jakob Diseases (CJD). Other characteristics of HSPs have also been studied by several scholars. These include binding of HSPs in vitro or in vivo directly to tau, facilitating microtubule polymerization and limiting tau aggregation and decreasing toxicity in vitro and in vivo [14]. HSPs can also bind to mutant HTT [15], alpha synuclein or prion oligomers. HSPs can also maintain quality control and can target abnormal or inactive proteins for degradation. HSPs are absorbed in different cellular stimuli and according to molecular size or function these are categorized as(i) The small HSPs (15 to 30 KD, HSP 10, HSP 26/27)(ii) Larger HSPs such as HSP 40, HSP 60, HSP 70, HSP 100/104/110 [16]These are mainly localized in cytoplasm and sometimes in nucleus. These are also found in mitochondria, endoplasmic reticulum (ER) etc. Under normal conditions, HSPs levels are properly regulated or maintained by local cellular environment but serious deterioration of HSPs defense system is observed when misfolded protein aggregates cross limit. Overproduction or over expression of certain HSPs can lead to development of some devastating diseases including cancer [17].As HSPs are induced by stressful conditions, two distinct conditions of induction may be mentioned like (i) auto induction in cells under any stress condition and (ii) induction due to administration of external stimuli that may be considered as stressful condition. Among these external stimuli, some Indian medicinal herbs are found to play vital role in this respect. There in are a good number of medicinal plants in India. These include (i) Bacopa Monnieri, (ii) Ashwaganda and (iii) Turmeric plant. HSPs induced by these plants are found to be suitable to disaggregate the aggregated parts of concerned proteins like alpha synuclein, Aβ amyloid or tau protein and HTT protein with respect to P.D, A.D and H.D respectively. A brief note on certain characteristics of Turmeric plant (Curcumina Longa) - the source of curcumin is mentioned here for the interest of the present work.
|
|
3. Curcumin – A Natural Polyphenol to Activate H.S. Response
- The scholars of the day are found to be interested to investigate natural polyphenol as HSP inducer to prevent neurodegenaration. This is because of the fact that these are basically nontoxic, safe, easily available, cost effective and also can be administreated orally. A good number of phenolic compounds derived from traditional medicinal plants have antioxidant, anti – inflammatory properties. But only few of these have been identified as HSPs inducers / modulators. Likewise curcumin derived from yellow powder / paste of turmeric – a product of the plant curcumina longa has recently been identified as HSPs inducers / modulators.Turmeric is used as an essential spice of Indian food item. On the other hand, it is being used as an item of therapeutic necessities in a number of diseases in Indian Ayurvedic system of medicine since more than five thousand years. It is used not only in India but also in China, Vietnam and other South – East Asian States. Practically, it is used as a therapeutic agent due to the presence of polyphenol in curcumin. But its use as therapeutic agent due to its pleotropic beneficial effects has started in Western World since last two decades only.From pharmacological point of view curcumin is anti inflammatory, anti oxidant and it also stimulates neurogenesis [18]. It has got anti – amyloid properties which reflect on its capability to be a potent drug for treatment of neurological diseases including A.D, P.D, depression, epilepsy, cerebral ischemia, brain tumor and different neuropathic pain. Due to its anti – amyloid properties, it can prevent aggregation of related protein (Aβ amyloid and tau isomers). Recently, it was shown by Maiti and Manna [19] that curcumin reduced HTT aggregation in CAG 140 KI mouse model of H.D. It was shown also by Yang et al [20] that curcumin reduced Aβ and tau aggregates from 3 x
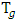 rat. In vitro data, it is shown that out of 214 – anti oxidant compounds, curcumin got strongest inhibitory effect on the formation of Aβ fibrils [21]. It reveals from recent experimental data that oral administration of curcumin could inhibit Aβ oligomerization and tau phosphorylation and thereby could improve impairment of AD – animal models [22]. It was also shown by other groups that tau vain injection of curcumin for one week had marked with 30% amyloid plaque size reduction in AD patients [23]. It can stimulate phagocytosis of Aβ in rat AD models [24]. It can decrease Aβ production by inhibiting GSK - 3β (the enzyme responsible for phosphorylation of tau). Further, curcumin is also found to bind with neurofibrillary tangles (NFT) in human AD and in animals too [25]. Moreover, it reveals from the studies of different group of scholars that curcumin can also bind with other amyloid proteins like β- pleated sheet structure including alpha synuclein protein [26], HTT [27] and also prion protein.
rat. In vitro data, it is shown that out of 214 – anti oxidant compounds, curcumin got strongest inhibitory effect on the formation of Aβ fibrils [21]. It reveals from recent experimental data that oral administration of curcumin could inhibit Aβ oligomerization and tau phosphorylation and thereby could improve impairment of AD – animal models [22]. It was also shown by other groups that tau vain injection of curcumin for one week had marked with 30% amyloid plaque size reduction in AD patients [23]. It can stimulate phagocytosis of Aβ in rat AD models [24]. It can decrease Aβ production by inhibiting GSK - 3β (the enzyme responsible for phosphorylation of tau). Further, curcumin is also found to bind with neurofibrillary tangles (NFT) in human AD and in animals too [25]. Moreover, it reveals from the studies of different group of scholars that curcumin can also bind with other amyloid proteins like β- pleated sheet structure including alpha synuclein protein [26], HTT [27] and also prion protein.4. Role of Huntingtin in Huntington
- It has been mentioned that like P.D, A.D etc, H.D is also caused due to aggregation of a protein called huntingtin abbreviated as HTT. Huntingtin gene is an “interesting transcript 15” gene symbol as IT 15 [28]. It is suggested by a number of scholars [28] that HTT plays vital role in long – term memory storage. It has got variable structure due to the presence of variable [28 i] number of glutamine residue (amino acid) - one of the basic constituents of the amino acid sequence of the protein HTT. In its normal form it contains 6 – 35 glutamine residues. But when an individual gets affected by H.D, the number of glutamine residue is increased from 36 residues to more than 100 based on which magnitude of HD attack may also be increased. The status of HD due to CAG repeats [28 ii] is described as
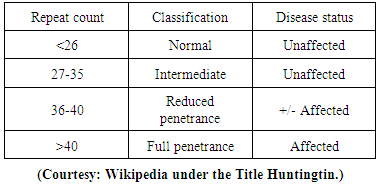 | Figure 1. Classification of the trinucleotide repeat, and resulting disease status, depends on the number of CAG repeats |
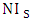 [29 i] are usually formed at the axons and dendrites of the nerve cells in specific regions of the human brain where damaged neuron characteristics of HD are produced. Subsequently, protease cuts HTT into smaller fragments which on entering the nerve cell nuclei form more clumps at the centrosomes. Thus NI causes problems to the cell and also brings significant change in the cell structure and also interferes with the normal production of other proteins. This leads to the fact that the formation of NI and outburst of neurodegenerative symptoms are found to be linked.Thus it reveals that the characteristics of HTT protein particularly with respect to aggregation, configuration etc is very complicated. Formation of aggregation in HTT protein is not as that of alpha synuclein protein or tau protein which get β aggregated with different segments of alpha synuclein protein and different isomers of tau protein particularly with respect to P.D and A.D respectively. The role of HTT in HD may be described as
[29 i] are usually formed at the axons and dendrites of the nerve cells in specific regions of the human brain where damaged neuron characteristics of HD are produced. Subsequently, protease cuts HTT into smaller fragments which on entering the nerve cell nuclei form more clumps at the centrosomes. Thus NI causes problems to the cell and also brings significant change in the cell structure and also interferes with the normal production of other proteins. This leads to the fact that the formation of NI and outburst of neurodegenerative symptoms are found to be linked.Thus it reveals that the characteristics of HTT protein particularly with respect to aggregation, configuration etc is very complicated. Formation of aggregation in HTT protein is not as that of alpha synuclein protein or tau protein which get β aggregated with different segments of alpha synuclein protein and different isomers of tau protein particularly with respect to P.D and A.D respectively. The role of HTT in HD may be described as 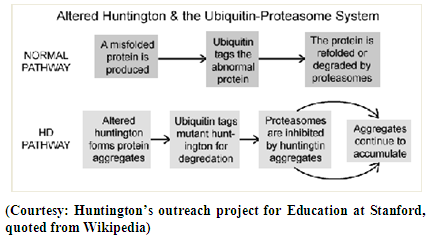 | Figure 2 |
5. Methodology
- In view of the above discussions, it requires an in-depth study to explore the complicacy of functioning of HTT protein particularly with respect to therapeutic aspects of H.D, based on which an alternative mechanism namely “protein vibration Approach” may be suggested for combating the problem with HSP induced by Indian Medicinal herb namely Turmeric plant. It is an established fact that protein vibrates due to either electrostatic force (potential) or due to external stimuli. The authors in a paper [30] suggested that the vibration characteristics of protein depend on the magnitudes of molecular weights of the concerned protein. It was also held by the authors that more the molecular weight of protein, the less is the number of vibration frequency. On the other hand, less the value of molecular weight of protein is, the more is the number of frequency. Thus the vibration pattern of protein may be described as
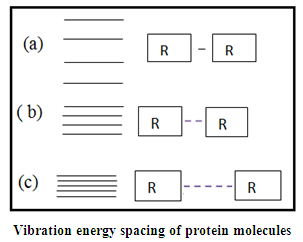 | Figure 3 |
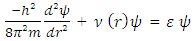 | (1) |
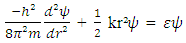 | (2) |
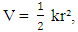 while other symbols stand for usual meaning.The energy of the allowed vibration states derived from the solutions of the Schrodinger’s Equation are
while other symbols stand for usual meaning.The energy of the allowed vibration states derived from the solutions of the Schrodinger’s Equation are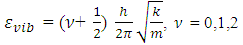 | (3) |
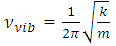 | (4) |
6. Result and Discussions
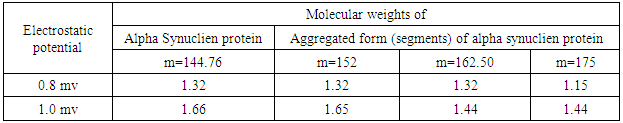
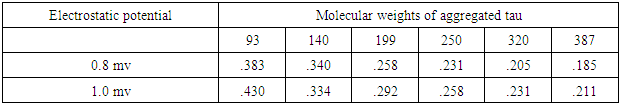
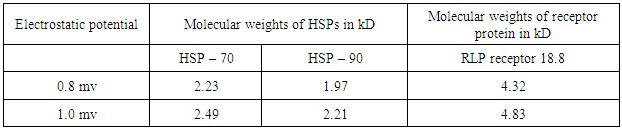
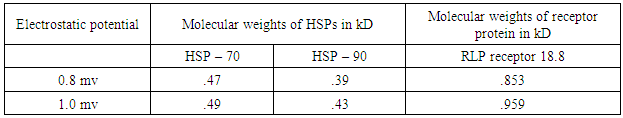
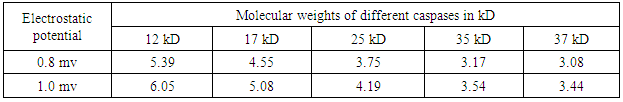
- Recently, a detailed study on the mechanism of functioning of the extract of Bacop Morrieri (B.M) and of Ashwagandha which are able to induce HSP – 70, 90 was undertaken from the view point of “Protein Vibration Approach” by the author [34] with respect to P.D and A.D respectively.Vibration frequencies and vibration energies of alpha synuclein protein (native) and its aggregated form responsible for P.D and those of tau (native) and it aggregated form with six isomers responsible for A.D were evaluated by using Equations (4) and (3) with electro static potential in the range of 0.8 mv to 1 mv and these are tabled in Tables 3, 4, 5 and 6.
|
|
|
|
|
|
|
|
7. Conclusions
- Our analytical approaches of disaggregation of aggregated HTT due to glutamine amino acids may be compatible to the experimental findings of Panchanan Maiti and others [19] where the scholars observed slow but progressing neurobehavioral improvements and reduction in HTT neuropil aggregates with curcumin administration to certain varieties of mice like CAG 140 KI (140 – glutamine codon) animal model HD. Further, our approaches with modulating effect of HSP – 70 and 90 are also found to be in parity with the investigated results of minimum dose (0’01μ M) of curcumin which was able to increase significantly HSP – 70 and HSP – 90 even after 24 hours of incubation in SH-SY5Y cells by the same group of scholars (unpublished). Thus curcumin might slow down amyloid formation and eventually will reduce neuronal death in neurodegenerative diseases.From the above observations, it may be held that out “Protein Vibration Approach” is on the one hand able to explain disaggregation of glutamine amino acid molecules from aggregated HTT responsible for HD and on the other hand is able also to modulate HSP in slowing down amyloid formation. Thus our analytical approaches are found to be in reasonable proximity with the experimental findings conducted by Panchanan Maiti and others [19].
ACKNOWLEDGEMENTS
- The author expresses his thanks to Dr. U.C. De, Asst. Professor, Department of Chemistry, Tripura University, Agartala, Tripura, for helpful discussion. He is thankful to Miss Joysree Sutradhar for under taking the Computer work of the Paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML