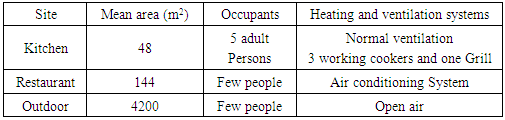
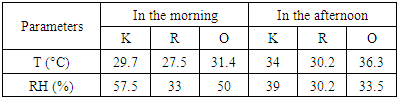
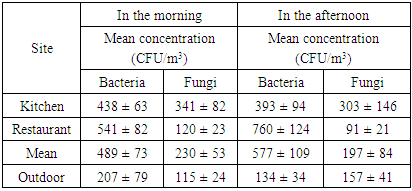
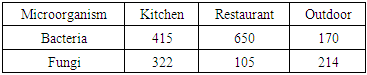
| [1] | U. Undochukwu, J. Inetianbor, F.O. Omorotionmwan, N.S. Okpuruka, Effects of high customer patronage on the indoor air quality of restaurants in Lokoja Metropolis and its public health impact, Am. J. of Microbiol. Res. 4 (2016) 51-55. |
| [2] | J. Manisha, R.K. Srivastava, Identification of indoor airborn microorganisms in residential rural houses of Uttarakhand, India, Int. J. Curr. Microbiol. App. Sci. 2 (2013) 146-152. |
| [3] | G. Bipasha, L. Himanshu, K. Rajesh, H. Naba, S. Arun, K.J. Vinod, Estimation of bioaerosol in indoor environment in the university library of Delhi, Sustain. Environ Res. 23 (2013)199-207. |
| [4] | S.P. Jozef, U. Kyaw Tha Paw, O.L. Danuta, W. Agnieszka, U. Krzysztof, Bacterial and fungal aerosols in indoor environment in upper Silesia, Poland, Atmos. Environ. 34 (2000) 3833-3842. |
| [5] | S. Teresa, M.G.M. Rosa, F. Alejandro, V.S. Jeronima, C. Jose, G. Mariano, Indoor airborne microbial load in Spanish university (University of Murcia, Span), Anales de Biologia, 31 (2009) 109-115. |
| [6] | L. Ulla, M. Teija, P. Tuula, et al., Microbial exposure symptoms and inflammatory mediators in nasal lavage fluid of kitchen and critical personnel in schools, Int. J. Occup. Med. Environ. Health, 18 (2005) 139-150. |
| [7] | E. Scott, Relationship between cross-contamination and the transmission of foodborne pathogen in the homes, Pediatr. Infect. Dis. J. 19 (2000) S111-S113. |
| [8] | K. Mattick, K. Durham, M. Hendrix, et al., The microbiological quality of washing-up water and the environment in domestic and commercial kitchens, J. Appl. Microbiol. 94 (2003) 842-848. |
| [9] | A. Medrano-Felix, C. Martinez, N. Castro-del Campo, et al., Impact of Prescribed cleaning and disinfectant use on microbial contamination in the home, J. Appl. Microbiol. 110 (2011) 463-471. |
| [10] | N. Kalogerakis, D. Paschali, V, Lekaditis, et al., Indoor air quality-bioaerosl measurements in domestic and office premises, J. Aerosol Sci. 36 (2005) 751-761. |
| [11] | P.N. Pegas, G. Evtyugina, C.A. Alves, et al., Outdoor/indoor air quality in primary schools in Lisbon: A preliminary study, Quim. Nova. 33 (2010) 1145-1149. |
| [12] | H. Aydogdu, A. Asan, O. M. Tatman, Indoor and outdoor airborne bacteria in child day – care centers in Edirn city (Turky), seasonal distribution and influence of 53-66. |
| [13] | G. Armadans, V. Rodriguez-Garrido, M. Gil-Cuesta, et al., Particle counting and microbiological air sampling: results of the simultaneous use of both procedures in different types of hospital rooms, Enferm. Infecc. Microbiol. Clin. 31 (2013) 217-221. |
| [14] | M.H. Seyed, N. Mahnaz, S. Kazuo, et al., Assessment of airborne particles in indoor environments: Applicability of particle counting for prediction of bioaerosol concentrations, Aerosol and air qual. Res. 16 (2016) 1910-2016. |
| [15] | Q. Hospodsky, D.J. Nazaroff, W. Yamamoto, et al., Human occupancy as a source of indoor airborne bacteria, Plosone 7 (2012) e34867. |
| [16] | M.P. Buttner, L.D. Stetsenbach, Monitoring of airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling, Appl. Environ. Microbiol. 59 (1993) 219-226. |
| [17] | W. Jones, K. Morring, P. Morey, et al., Evaluation of the Andersen viable impactor for single samling, Am. Ind. Hyg.Ass. J. 46 (1985) 294-298. |
| [18] | C.F. Green, P.V. Scarpino, The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria, Environ. Engineer Policy. 3 (2002) 101-107. |
| [19] | E.F. Gilberto, T.B. Scott, J.G. Caporaso, et al., Diversity, distribution and sources of bacteria in residential kitchens, Environ. Microbiol. 15 (2013) 588-596. |
| [20] | S. Chunxiao, L. Josephine, Y. fang, A case study of upper-room UVGI in densely-occupied elementary classrooms by real-time fluorescent bioaerosol measurements, Int. J. environ. Res. Public Health. 14 (2017) 51-63. |
| [21] | M. Frankel, G. Beko, M. Timm, et al., Seasonal variations of indoormicrobial exposures and their relation to temperature, relative humidity, and air exchange rate, Appl. Environ. Microbiol. 78 (2012) 8289-8297. |
| [22] | J. Zhau, W. Fang, Q. Cao, et al., Influence of moisturizer and relative humidity on human emissions of fluorescent biological aerosol particles, Indoor air. 27 (2017) 587-598. |
| [23] | A. K. Jain, Survey of bioaerosol in different indoor working environments in central India, Aerobiologia. 16 (2000) 221-225. |
| [24] | L. Chih-Shan, H. Po-An, Bioaerosol characteristics in hospital clean rooms, The Sci. Tot. Environ. 305 (2003) 169-176. |
| [25] | T. Soto, M. Lozano, J. Vicente-Soler, et al., Microbiological survey of the aerial contamination in urban areas of the city of Murcia, Spain, Anales de Biologia. 31 (2009) 7-13. |
| [26] | J.H. Lee, W.K. Jo, Characteristics of indoor and outdoor bioaerosols at Korean high-rise apartment buildings, Environ. Res. 101(2006) 11-17. |
| [27] | M. Sibel, A. Munevver, Y.R. Abbas, et al., Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey, Clean. 37 (2009) 487-493. |
| [28] | World Health Organization, Indoor Air Quality: Biological Contaminants. Copenhagen: WHO Regional Publications, European Series No.31 (1990). |
| [29] | W.K. Jo, Y.J. Seo, Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools and homes, Chemosphere. 61 (2005) 1570-1579. |
| [30] | P.A. Scheff, V.K. Paulius, L. Curtis, et al., Indoor air quality in a middle school, Part II: development of emission factors for particulate matter and bioaerosols, Appl. Occup. Environ Hyg. 15 (2000) 835- 842. |
| [31] | W.C. Hinds, Aerosol Technology, Properties, Behavior, and Measurement of Airborne Particles. 2nd edn. Wiley, New York. 1999. |
| [32] | A. Fox, W. Harley, C. Feigley, et al., Large particles are responsible for elevated bacterial marker levels in school air upon occupation, J. Environ. Monit. 7 (2005) 450-456. |
| [33] | D. Hospodsky, N. Yamamoto, W.W. Nazaroff, et al., Characterizing airborne fungal and bacterial concentrations and emission rates in six occupied children's classrooms, Indoor Air. 25 (2015) 641-625. |
| [34] | M. Moustafa, Concentration and size distribution of biological particles in school classrooms, J. Adv. Phys. 13 (2017) 5085-5091. |
| [35] | T. Meklin, T. Reponen, M. Toivola, et al., Size distributions of airborne microbes in moisture-damaged and reference school buildings of two construction types, Atmos. Environ. 36 (2002) 6031-6039. |
| [36] | R.L. Gorny, T. Reponen, S.A. Grinshpun, et al., Source strength of fungal spores aerosolization from moldy building material, Atmos. Environ. 35 (2001) 4853-4862. |
| [37] | S.C. Christopher, M.W. Christopher, Bioaerosols Handbook, CRC Press. 1995. |
| [38] | G. Dexing, G. Chunmei, L. Yanju, et al., Study on the concentration and distribution of the airborne bacteria in indoor air in the lecture theaters at Tianjin Chengjian university, China, Proc. Eng. 121 (2015) 33-36. |
| [39] | J.M. Seltzer, Biologic contaminants, Occup. Med.State of the art review. 10 (1995) 1-25. |


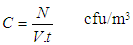 Where: C is the particles concentration,N is the number of colonies on each stage of the impactorV is the impactor flow rate (m3/h),t is the sampling time (hour)
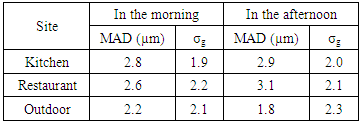
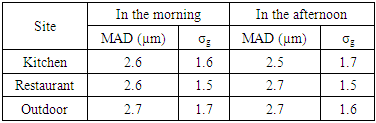
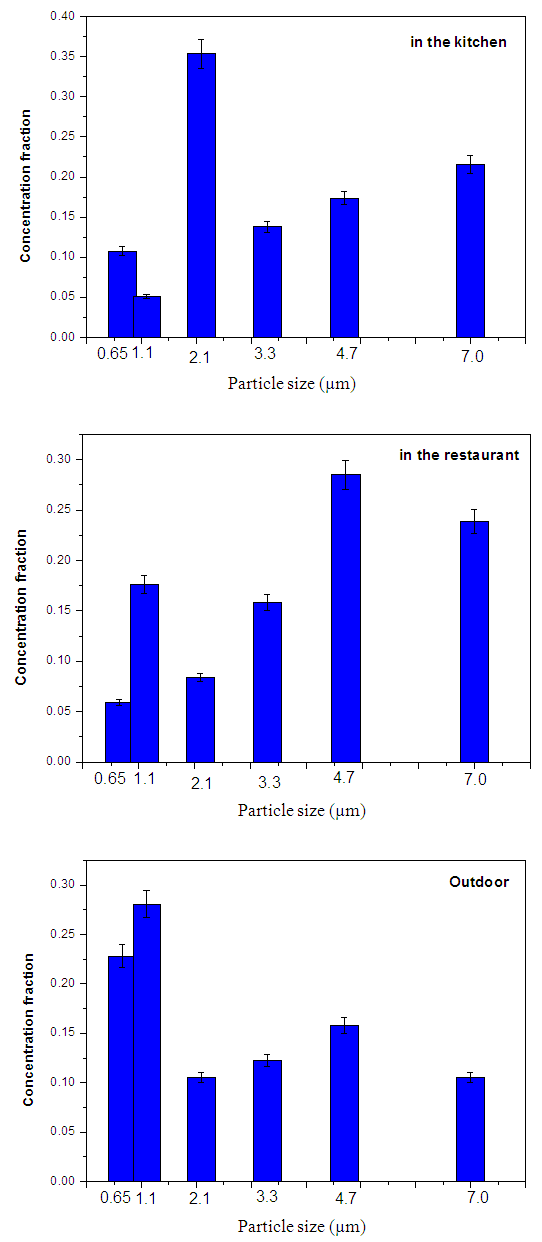
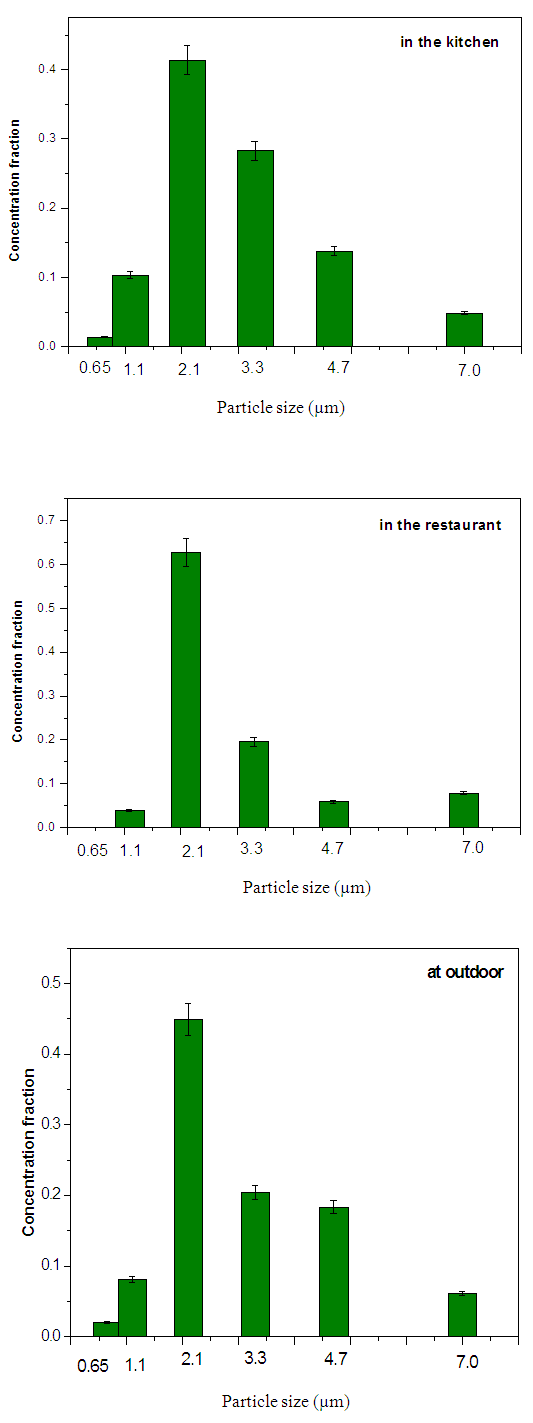
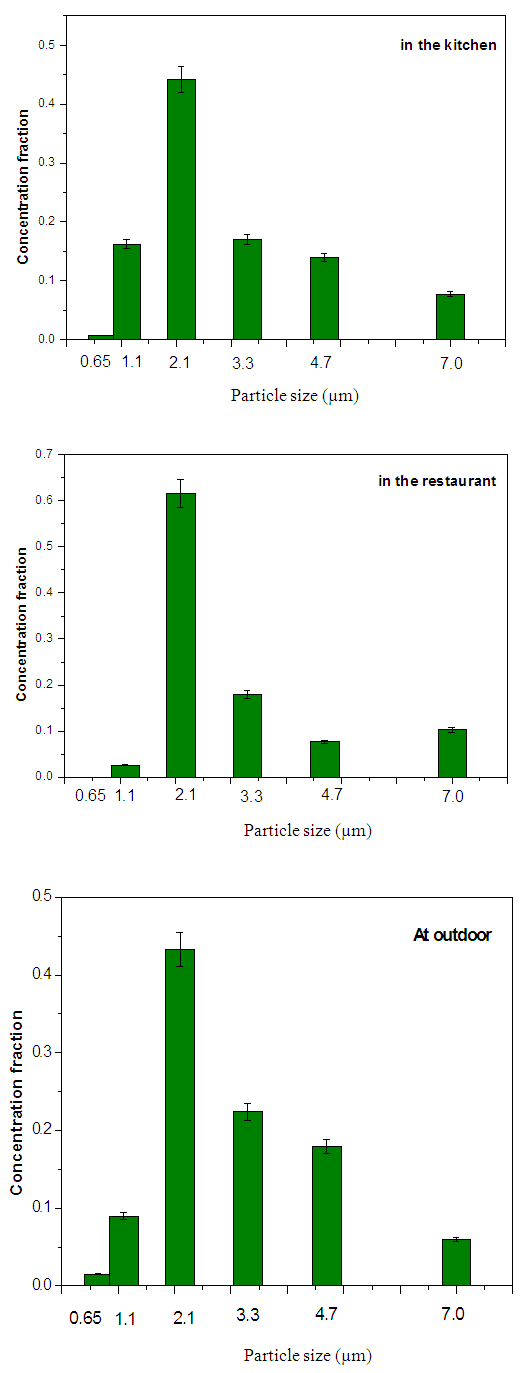
Where: C is the particles concentration,N is the number of colonies on each stage of the impactorV is the impactor flow rate (m3/h),t is the sampling time (hour)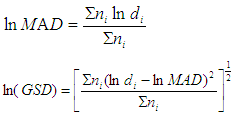 Where MAD is the median aerodynamic diameter, ni is the fraction in stage i, di is the cutoff diameter of the stage i and GSD is the geometric standard deviation (σg). MAD is defined as the diameter at 50% cumulative fractions. GSD of the size distribution is defined as the diameter at 84% cumulative number divided by the diameter obtained at 50%.Size distribution of bacteria sampled in the kitchen, restaurant and outdoor in the morning and afternoon are shown in figures 1 and 2, respectively. The size distribution parameters of bacteria; median aerodynamic diameter (MAD) and geometric standard deviation (σg) are summarized in table 6.
Where MAD is the median aerodynamic diameter, ni is the fraction in stage i, di is the cutoff diameter of the stage i and GSD is the geometric standard deviation (σg). MAD is defined as the diameter at 50% cumulative fractions. GSD of the size distribution is defined as the diameter at 84% cumulative number divided by the diameter obtained at 50%.Size distribution of bacteria sampled in the kitchen, restaurant and outdoor in the morning and afternoon are shown in figures 1 and 2, respectively. The size distribution parameters of bacteria; median aerodynamic diameter (MAD) and geometric standard deviation (σg) are summarized in table 6.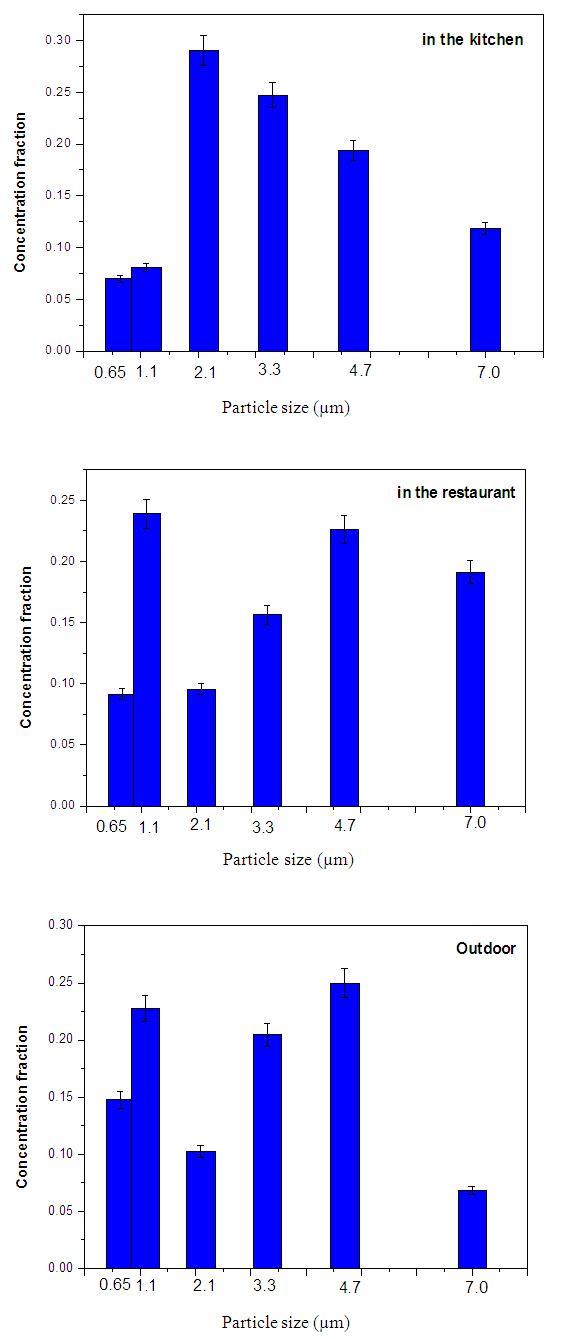
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML