-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2017; 7(3): 41-47
doi:10.5923/j.biophysics.20170703.02

Role of Protein Vibration in Anti-Alzheimer’s Effect of Ashwagandha (Withania Symnifera) - An Analytical Approach
Brajagopal Majumder
Retired Reader in Physics, Govt. Degree College, Agartala, Tripura, India
Correspondence to: Brajagopal Majumder, Retired Reader in Physics, Govt. Degree College, Agartala, Tripura, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Alzheimer normally occurs in old age people. This results in memory loss and cognitive decline. The patient at his crucial stage losses almost his physical fitness. The disease has got no complete cure till the death. It is due to aggregation of either a different tau isomers or different constituents of Aβ – amyloid protein. A good number of scholars are now – a – days conducting a study on therapeutic aspects of Ashwagandha – a traditional Indian herb. Empirical studies also reflect on a successful implication of Ashwagandha root extract on Alzheimer’s affected mice. But the mechanism of activity of the extract has rarely been explained. The present paper focuses on the mechanism of disaggregation of aggregated protein by HSP – 70 induced by the extract of Ashwagandha and also on the removal technique of the disaggregated fragments with the help of LRP receptor. The problem has been viewed in the context of Protein Vibration – a hypothetical approach of the physical characteristics of protein.
Keywords: Tau proteins, Aβ - amyloid, LRP receptor, Ashwagandha extract, Heat Shock Protein, Aggregation and Disaggregation
Cite this paper: Brajagopal Majumder, Role of Protein Vibration in Anti-Alzheimer’s Effect of Ashwagandha (Withania Symnifera) - An Analytical Approach, International Journal of Biophysics , Vol. 7 No. 3, 2017, pp. 41-47. doi: 10.5923/j.biophysics.20170703.02.
Article Outline
1. Introduction
- Alzheimer is a class of neurodegenerative disease. The disease is marked by memory and judgement loss. It usually occurs in older people of 65 years of age. Basically two types of proteins - Aβ amyloid and tau protein are responsible for Alzheimer disease. The paper focuses on the role of tau protein aggregation in A.D along with a brief functioning of Aβ amyloid plaques.During the later part of 1970, a number of scholars investigated the fact that microtubule associated protein tau promotes microtubule assembly and stability in the nervous system [1]. It was also observed by the scholars that multiple isomers are generated from a single gene through alternative RNA splicing. This leads to cell type specific expression of different forms [2].Tau protein is found to be associated with axonal microtubules in a normal cell [3], but in case of A.D it is immobilized in the somatodendritic component of the nerve cell. Here it is found to form part of the neurofibrillary tangles (NFT) [4]. NFTs are aggregates of filamentous tau polymers. These comprise a protein of the fibrillar pathologies of A.D. These increase tau density with regional and global accepts of cognitive decline [5-7]. It is noted in early annals of tau in Aβ that the molecule gets truncated [8, 9] in many NETs. Practically, this is an alteration in tau. From these a simple question arises how tau polymers form and get evolve during the course of A.D?Tau is found to undergo substantial conformational changes prior and after polymer formation. This paper will reflect on the recent findings on folding and proteolytic truncation with respect to molecular and structural alternations of this protein during A.D pathogenesis. Thus it can be held that it is tau filament formation which causes neurodegenerative diseases. In short, it is observed that in case of A.D tau accumulates within neurons and forms thread which twist abnormally and form tangles. This creates hindrance to the transportation of nutrients and other essential materials resulting in brain cell death which cause a variety of effects including A.D in human.
2. Tau Conformation and Tangle Formation
- In order to form filaments tau is found to undergo a massive conformation change to begin with aggregation process. The extent of this change was appreciated with discovery of Alz50 monoclonal antibody [4]. This antibody binds to tau when its amino terminus comes in contact with the microtubule binding repeats (MTBR). This mode of conformational change from native tau to tau 66 as suggested by Lester I. Binder and others [3] is diagrammatically shown in. Fig. 1.
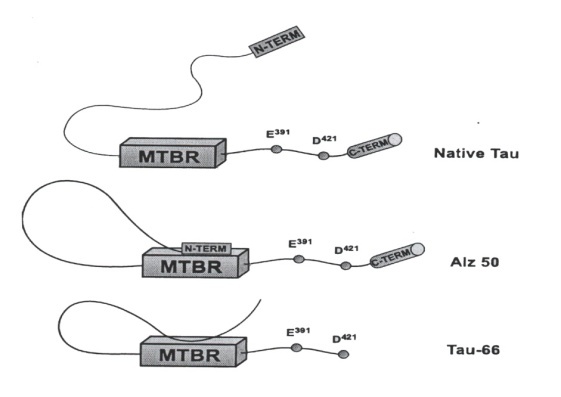 | Figure 1. Diagrammatic representation of the conformational states of tau in NFTs. (Curtsy Lester I. Binder et al, 1985) |
3. Protein Accumulation in A.D.
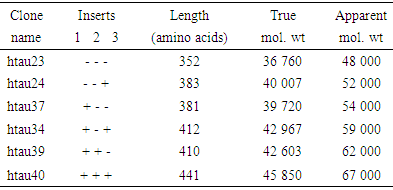
- Six cloned isomers of human microtubule associated tau proteins were observed by M. Goedert and R Jakes [10] in escherichia coli. These were purified by them in a biologically active form. The amino acid present varies from 352 to 441 in length and differ from each other by three or four repeats in the carboxy terminal half and by presence and absence of 29 or 58 amino acid inserts in the amino terminus. It was reported by Flament and Delacourte [11] that there are abnormally phosphorylated forms of tau that lead to a shift in mobility in brain tissue from patients with A.D. Practically speaking, neurodegenerative diseases are characterized by protein aggregate formation in the brain. This is due to misfolding of different proteins. A number of irregular incidents are caused by these clumps of protein. These are (i) damage and death of brain cell (neuron) (ii) loss of synapse or (iii) disturbance in interconnectivity between nerve cells. Thus based on the observation of Flament and Delacourte [11] it is held that in case of A.D tau protein fragments accumulate outside the nerve cells, build plaques and spherical clump which ultimately damage the brain cells. In short it reveals that A.D. is caused due to aggregation of different isomers of tau proteins having apparent molecular weights of 48- 67 kD by proteolytic process. On SOS – page gels six isomers of tau having apparent molecular weights are named [12] as(i) 48 kD (three repeats containing form), (ii) 52 kD (four – repeat containing form) (iii) 54 kD (three – repeat containing form with long amino terminal insertion), (iv) 59 kD (four – repeat containing form with short amino terminal insertion) (v) 62 kD (three repeat containing form with long amino terminal insertion) and 67 kD (four repeat containing form with long amino terminal insertion). When these are mixed together, a set of six bands runs on SDS – page gels with apparent molecular weight (48 – 67) kD with a characteristic pattern of spacing.On the other hand, fragment of beta amyloid 42 proteins accumulate outside the nerve cell, build up amyloid plaque which eventually leads to death of nerve cells. Moreover, it is to be noted that LRP (Low – density lipoprotein receptor – related protein) is also linked to A.D. Demonstration on LRP by different scholars contributes to Aβ generation. Thus β– amyloid peptide is regarded as essential component of senile plaques. Thus Aβ is derived from β amyloid precursor protein (APP). Different demonstrations established the fact that LRP, a member of LDL (Low density Lipoprotein) receptor family binds and mediates the endocytosis of APP isomers containing KPI (Kunitz proteinase inhibitor) domain. Thus it reveals that LRP is a multifunctional endocytic receptor abundantly expressed in liver and brain. This in turn mediates the hepatic uptake of circulating chylomicron remnants, scrpin enzyme complexes and proteinases of fibrinolytic pathway. Thus it is held that LRP plays a role in the path biology of A.D by facilitating the delivery of cell surface APP to endosomal compartment where Aβ peptic can be generated. On the otherhand, LRP in liver removes β– amyloid from blood plasma and keeps it away from entering the brain. This constitutes the basic principle of treating A.D.
4. Role of Ashwagandha in A.D.
- It is reported by the scientists of National Brain Research Centre (NBRC), New Delhi that an extract of Ashwagandha root administered on mice brought from Jackson Labs in US with A.D. could reverse memory loss of the mice. Basing on these findings scientists assumed that administration of Ashwagandha root extract may be effective for the cure of A.D in humans also.V. Ravindranath and others [13], a neuroscientist of NBRC conducted an experiment at Delhi University on genetically modified mice with A.D. Two sets of mice – middle aged (9 – 10) months and old aged (2) years were administered oral doses of the extract for 30 days and these mice were kept under monitoring. After one month, the scientist found a reduction in amyloid plaques (a symptom of A.D) in mice brain and also found improvement in cognitive abilities. The scholars [13] held the view that “the mice had Alzheimer and they were not able to learn or retain learning. But astonishingly after 20 days of extract administration they used to behave differently and after 30 days of administration they had started behaving normally”. They also added that “the extract did not work directly but it enhanced a protein in the blood and acts like a sponge to pull out the amyloid from the brain”.It is now an established fact that with biologically active steroids Ashwagandha – an Indian herb fights free radicals and is able to increase the activity of the natural antioxidants in the body and also to reduce inflammation – a common factor in A.D. Ashwagandha has withanolide A that can reconstruct the disturbed neuronal networks and synapses with the process of generating axons and dendrites. In this way it can improve the memory deficits [14].They explained the phenomenon of pulling out amyloid from the brain with a protein in the liver which is enhanced due to administration of Ashwagandha root extract and which acts like a sponge. The protein as suggested by the authors may be termed as LRP receptor protein since it is discussed that LRP can remove beta – amyloid in blood plasma. This may be considered as one of the treating modes of A.D. However, sponging can occur after disaggregation of aggregated proteins. Moreover, the sponging effect of amyloid molecule as mentioned by the scholars [13] is based on the change of behavioral attitudes of mice after 30 days of administration of Ashwagandha. However this is supported by pathological Experiment with blood plasma of the affected mice. This paper focuses on the alternative approach of treating the problem. Ashwagandha root extract in addition to enhancing protein receptor in Liver, can also induce [15] Heat Shock Protein. (HSPs). These proteins are used as (i) up regulation in stress and (ii) as chaperone. Recent development in HSPs a chaperone with respect to both immunity and therapeutic aspects attracts the scholars much. Here we like to concentrate our attention to the disaggregation of tau protein isomers which are aggregated through proteolytic process with HPS 70 or 90 induced by Ashwagandha root extract when it is administrated to an A.D patient.In a recent paper, the author [16] explained analytically the role of HSP – 70 induced by Bacopa Monnerie – an Indian herb in disaggregation of aggregated alpha synuclein protein responsible for Parkinson Disease (P.D) in the context of his proposed “Protein Vibration” approach. A similar attempt is taken here to investigate analytically the anti Alzheimer’s effect of Ashwagandha in the context of “Protein Vibration”. Though the basic principle of Alzheimer’s and Parkinson’s disease is aggregation of protein namely tau (as we are considering) and alpha synuclein responsible for the two diseases respectively, yet the necessity of studying A.D. separately lies in the fact that functioning of tau responsible for A.D involves a number of complicated steps which have already been discussed. Similarly, a good number of papers [17, 18] with cognitive loss problems due to diabetic and other form of mental disorders have been published in the context of “Protein Vibration”.
5. Methodology
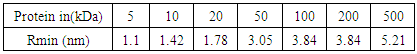
- In view of the above discussion, an attempt has been taken here to examine the functioning of HPs with respect to therapeutic effect of Ashwagandha – an Indian herb traditionally used for recovery of a number of diseases in the context of Protein Vibration. It is an established fact that protein vibrate due to either electrostatic force (potential) or due to external stimuli. In a paper [19] the authors suggested that the vibration characteristics of protein depend on the magnitudes of molecular weights of the concerned protein. The vibration frequency and vibration energy of the concerned protein can be evaluated by using one dimensional Schroclinger’s Equation. The reason of considering one dimensional Schrodinger’s Equation lies in the fact that an electrostatic force can control atomic motion in a protein. For a protein [20] this can be divided into two components – a large and a small.Conformational substrates for the protein in its native state may correspond to the same position ‘r’ of the particle at time ‘t’. Thus r (t) can be split into two independent components.
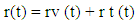 Where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation
Where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation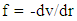 | (1) |
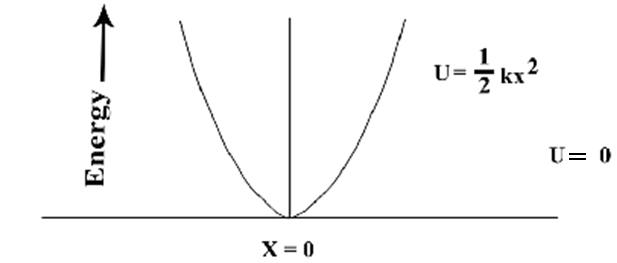 | Figure 2. Oscillation of vibrating particle |
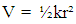 | (2) |
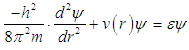 | (3) |
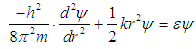 | (4) |
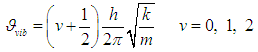 | (5) |
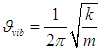 | (6) |
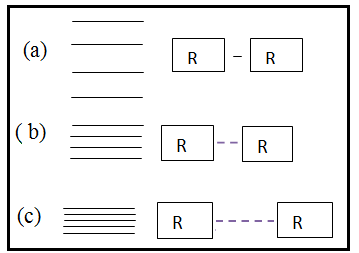 | Figure 3. Vibration energy spacing of protein molecules |
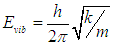 | (7) |
6. Results and Discussions
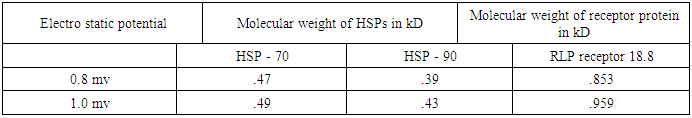
- Basically two type of protein namely Aβ amyloid and tau are responsible for A.D. This paper focuses on the role of isomers of tau proteins along with responsible receptor proteins. These are listed in Table 1. Identity of tau isomers [12]
|
|
|
|
|
|
|
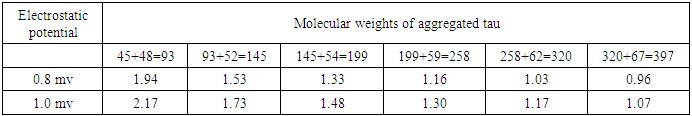
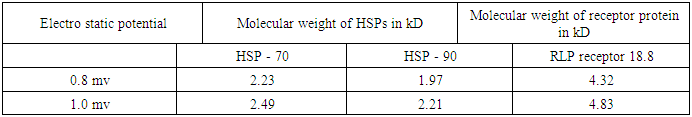
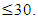 For the sake of the problem, this has been considered in between (0.8 to 1) mv with respect to variable pH. It reveals from Table (3) that both the vibration frequencies and vibration energies of native tau is getting decreased with increase in molecular weight by proteolytic process of aggregation of different isomers. But the vibration frequency and vibration energy of HSP – 70 are higher than those of aggregated tau. Thus it is held that HSP – 70 is able to disaggregate different isomers of aggregated tau. Practically it is expected that isomer wise disaggregation occurs due to higher magnitude of vibration frequency and vibration energy of HSP – 70. This means higher magnitude of vibration frequency and vibration energy of HSP – 70 in comparison to those of aggregated proteins are able to undertake disaggregation of aggregated proteins isomer (segment) wise. Thus it is found that higher values of vibration frequency and vibration energy either can disaggregate the aggregated molecule of other proteins (molecular weights are higher than those of HSP -70) or can change the signalling pathway of other proteins (molecular weights are lower than those of HSP – 70). Thus in can of A.D, HSP – 70 induced due to administration of root extract of Ashwagandha can disaggregate the aggregated tau isomer (segment) wise and as a result a total amount of 342 kD of tau isomers will be disaggregated in each cycle.On the other hand, molecular weight of LRP receptor is 18.8 kD. So, it will vibrate more frequently than any other proteins associated in case of A.D. On administration of Ashwagandha root extract as Ravindranath [13] held, the enhanced molecule of proteins (LRP) will be able to pull out the disaggregated isomers of tau or disaggregated constituents of Aβ – amyloid by virtue of its higher vibration frequencies and vibration energies. This means, cure of A.D patients depends on two vital issues.(i) Disaggregation of aggregated isomers of tau proteins or different constituents of Aβ. – amyloid by HSP – 70 induced even by oral administration of Ashwagandha root extract.(ii) Pulling out of these disaggregated segments by LRP receptor that acts like a sponge [13] on oral administration of Ashwagandha.
For the sake of the problem, this has been considered in between (0.8 to 1) mv with respect to variable pH. It reveals from Table (3) that both the vibration frequencies and vibration energies of native tau is getting decreased with increase in molecular weight by proteolytic process of aggregation of different isomers. But the vibration frequency and vibration energy of HSP – 70 are higher than those of aggregated tau. Thus it is held that HSP – 70 is able to disaggregate different isomers of aggregated tau. Practically it is expected that isomer wise disaggregation occurs due to higher magnitude of vibration frequency and vibration energy of HSP – 70. This means higher magnitude of vibration frequency and vibration energy of HSP – 70 in comparison to those of aggregated proteins are able to undertake disaggregation of aggregated proteins isomer (segment) wise. Thus it is found that higher values of vibration frequency and vibration energy either can disaggregate the aggregated molecule of other proteins (molecular weights are higher than those of HSP -70) or can change the signalling pathway of other proteins (molecular weights are lower than those of HSP – 70). Thus in can of A.D, HSP – 70 induced due to administration of root extract of Ashwagandha can disaggregate the aggregated tau isomer (segment) wise and as a result a total amount of 342 kD of tau isomers will be disaggregated in each cycle.On the other hand, molecular weight of LRP receptor is 18.8 kD. So, it will vibrate more frequently than any other proteins associated in case of A.D. On administration of Ashwagandha root extract as Ravindranath [13] held, the enhanced molecule of proteins (LRP) will be able to pull out the disaggregated isomers of tau or disaggregated constituents of Aβ – amyloid by virtue of its higher vibration frequencies and vibration energies. This means, cure of A.D patients depends on two vital issues.(i) Disaggregation of aggregated isomers of tau proteins or different constituents of Aβ. – amyloid by HSP – 70 induced even by oral administration of Ashwagandha root extract.(ii) Pulling out of these disaggregated segments by LRP receptor that acts like a sponge [13] on oral administration of Ashwagandha. 7. Conclusions
- From the above discussions, it is held that the main cause of A.D is aggregation of either tau isomer or different constituents of Aβ – amyloid. So, the first step of therapeutic activity lies in disaggregation of aggregated tau or Aβ – amyloid proteins.It reveals that HSP – 70 by its characteristics function as intra – cellular chaperones for aggregated tau or Aβ – amyloid proteins. In the above discussion, it is shown that HSP – 70 helps in disaggregating the aggregated tau protein and LRP receptor is able to pull out these disaggregated segments by acting as sponge. All these happen as the author suggests, due to higher magnitudes of vibration frequency and vibration energy of HSP – 70 and LRP receptor generated by electrostatic potential considered arbiter in the range of (0.8 to 1.0) mv. The cause of pulling out of disaggregated isomers or different constituents of Aβ amyloid protein as suggested by Ravindranath [13] has also been discussed taking into account the “Protein Vibration Approach”.However, the pulling out phenomenon of disaggregated isomers as suggested by the scholars [13] may be verified by conducting blood plasma test of A.D patient where the molecular weight of aggregated tau or Aβ – amyloid would definitely be decreased after regular administration of Ashwagandha root extract. A sophisticated pathological Lab may be able to conduct this test and observe the differential between initial (affected) and final (after affected) conditions of the patients.On the other hand, responses of tau or Aβ – amyloid protein in case of both aggregated and disaggregated forms may also be tested from the initial and final (after a few days of Ashwagandha extract application which induces HSP – 70) recordings of EEG technique with beta wave having amplitudes of 20 – 25 microvolt and initial frequency 13 – 30 Hz.
ACKNOWLEDGEMWNTS
- The author is thankful to Dr. U.C. Dc, Associate Professor, Dept. of chemistry, Tripura University, Agartala, India for helpful discussion. The author is also thankful to Miss. Joysree Sutradhar for undertaking the Computer work of the paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML