-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2017; 7(1): 8-15
doi:10.5923/j.biophysics.20170701.03

An Analytical Approach to Modulating Effects of Heat Shock Proteins towards Immune Responses of Cancer in the Context of Protein Vibration
Brajagopal Majumder
Former Reader in Physics, Govt. Degree Collage, Agartala, India
Correspondence to: Brajagopal Majumder , Former Reader in Physics, Govt. Degree Collage, Agartala, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A brief account on cancer – one of the devastating diseases of the world has been outlined here. Cancer being a genetic disease arises from accumulation of mutations in critical genes. There are no unilateral reasons for its development. However, some proteins have by this time been identified for the development of cancer in human body. On the other hand, some proteins namely Heat shock proteins (Hsp) are induced either in different cancer affected cells or induced due to external administration of therapeutic agents including Indian medicinal herbs like Bacopa Monnicri etc. These are now found to modulate immune system by stimulating both innate and adaptive responses. Hsp – based therapeutic in cancer trials are also now available. One Hsp – band Vaccine Vitespen by name, made of Hsp gp - 96 is now licensed for use. This paper focuses on the dynamism of the use Hsp – 70, Hsp gp - 96 in the context of protein vibration approach due to electrostatic potential of the cell in the range of (0.8 to 1.0 and 25) mv. The potential range has arbitrary been choosen for sake of the paper. The paper also focuses on the process of modulation of the immune system by Hsp – 70 and gp – 96 basing on the frequency generated by the respective Hsp.
Keywords: Cargo Proteins, Oncogene, Protein Receptors, Heat Shock Proteins, Protein Vibration, Modulation, Immunogenicity
Cite this paper: Brajagopal Majumder , An Analytical Approach to Modulating Effects of Heat Shock Proteins towards Immune Responses of Cancer in the Context of Protein Vibration, International Journal of Biophysics , Vol. 7 No. 1, 2017, pp. 8-15. doi: 10.5923/j.biophysics.20170701.03.
Article Outline
1. Introduction
- Cancer is one of the most devastating diseases in the world. It is numerous in nature. The available number at present amounts to 200 approximately. The cause of the disease is not unilateral. The scholar’s cannot still specify the particular causes for the disease. The actual reason of attacking with the disease is also unidentified. And also the age of attacked person is uncertain. An individual can be attacked at any age. In this crucial juncture, it is held that uncontrolled cell growth or cell division may be considered as development of cancer cells. The essential alterations as identified by the scholars (1) for malignant growth include the followings(i) Self sufficiency in growth signals (ii) Insensitivity to growth inhibitory signals (iii) Evasion of programmed cell death(iv) Limitless replicative potential(v) Sustained angiogenesis (vi) Tissue invasion and metastasis From all these parameters it is held that “cancer arises from accumulation of mutations in critical genes”.Since proteins constitute the major constituents of the living beings, proteins of different kinds may be held responsible for the cause and cure of cancers. Different types of proteins are responsible for different varieties of cancers. The study of the researchers in Felsher’s Laboratory (2) focuses on the role of Myc protein. Myc protein is encoded by a gene known as oncogene. Oncogene being a vital cellular functionary becomes powerful cancer proteins when they are mutated or expressed incorrectly. The Myc oncogene is found to be mutated or misregulated in nearly half of human cancers. The researchers of the same Lab. also studied on a particular phenomenon known as “oncogene expression addiction” which showed that tumor cells are completely dependent on the expression of oncogene. Thus Myc causes an increase in the levels of proteins that promote cell division with the ability of outwitting the immune molecules. The dean (2) of Felsher Lab. described the causal connection between the mode of causing cancer cells to evade the immune system. Thus this constitutes the basic principal of oncogene expression and development of cancer in animals.In case of human genome, Myc is located on chromosome 8 and it is believed to regulate expression of 15% of all genes through binding on enhancer box sequences (E – boxes). A mutated version of Myc is found to be expressed in many cancers. Hence, it is held that Myc is a strong proto – oncogene and is very often seen to be unregulated in many types of cancers. Myc over expression stimulates gene simplification which causes several types of cancers like breast, colorectal, pancreatic, gastric, uterine etc. Similarly, other oncoproteins like mutated P53 - the most devastating one, may appear during carcinogenesis. The mutated and conformation ally altered proteins become expressed or over expressed in cancer / tumor cells. In addition heat shock proteins (Hsp) of different molecular weights are also found to be associated with proteins responsible for causing cancers. For example, Hsp -27 is found to be associated with Erά in female breast cancers and Hsp – 70 is found to be associated with Erά in breast cancers.
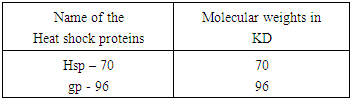
2. A Brief Note on the Role of Heat Shock Proteins
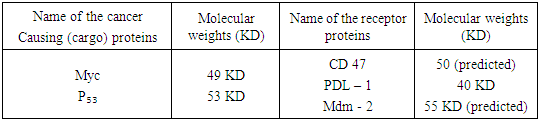
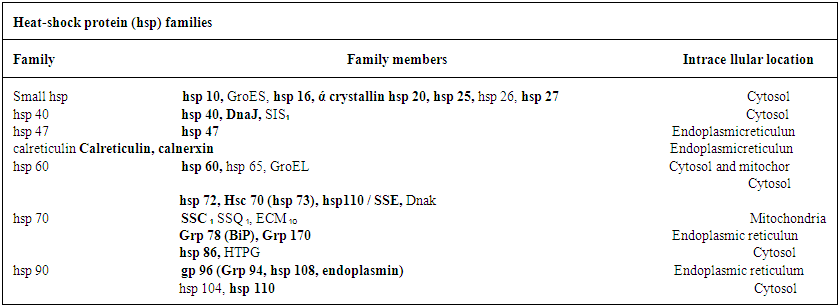
- It is now an established fact that some proteins produced in respective cells are exposed to stressful conditions. These are known as Heat shock proteins (Hsp). These proteins are better used as (i) up regulation in stress and (ii) as chaperones. Recent development in Hsps as chaperones with respect to both immunity and therapeutic aspects attracts the scholars much.Heat shock proteins (Hsp) as the scholars held, are found to carry on antigenic profile or fingerprint of the cells from which they are derived, possess adjuvant activity and bind to receptors on DC to promote their respective functioning (3). At present a good number of Heat shock proteins have been identified. These are now grouped in several families. These are named in accordance with the molecular weights of family members (3) asWhile Hsp – 90, Hsp – 70, Hsp – 60 / chaperone and Hsp – 40 families have been identified in man, gro EL (Hsp – 60), Dnak (Hsp – 70) constitute as members of main Hsp families in prokaryotes (4). Heat shock proteins are induced only under stress conditions in normal cells. Stress condition means protein are getting denatured by heat shock, oxidative stress and other protein damaging events. Under this condition, Hsps are expected to modify the structure and interactions of other proteins as molecular chaperones. It was held by a number of scholars that Hsp – 27, Hsp – 70, Hsp – 90 and Hsp – 110 are found to be dominantly expressed proteins after stress. Studies conducted by the scholars have shown that Hsp – 10 and Hsp – 60 complexes are able to mediate protein folding while Hsp – 70 and Hsp – 90 are involved in both generic protein folding pathways and also in specific association with key regulatory proteins within the cell. On the other hand, Hsp – 90 plays a pivotal role in cell regulation, forming complexes with cellular kinesis, transcription factors and other molecules.With respect to functioning as intracellular protein chaperones, Hsps modulate the immune system by stimulating both innate and adaptive responses. Usually Hsps are found to undertake the functioning as chaperones and as cytokine. Being released from a host or pathogen cell, Hsps bind to cellular receptors and trigger an innate immune response. It also includes pro- inflammatory cytokines and chemokines. This results in processing of cargo proteins to be carried by Hsp along with and acquired immune responses to pathogens leading to the functioning as vaccine adjutants with respect to infections and cancers. A number of receptors which play a vital role in Hsp binding are shown (3) in table 2.
 | Table 1 |
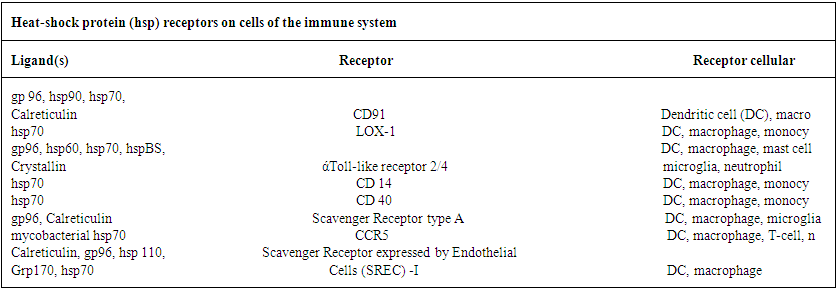 | Table 2 |
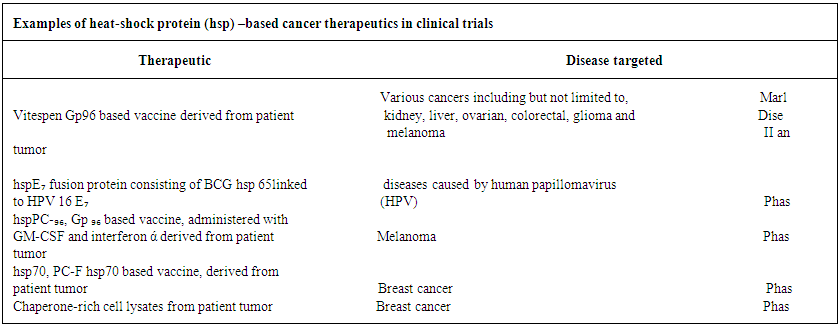 | Table 3 |
3. Methodology
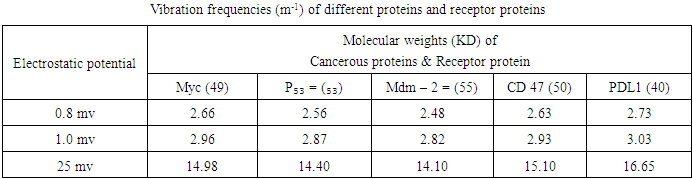
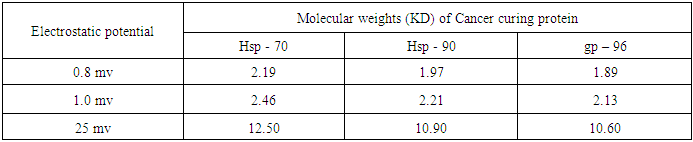
- In view of the above discussions, an attempt has been taken here to examine the functioning of Hsps with respect to immune system and therapeutic aspects of cancer / tumor cells in the context of protein vibrations. It is an established fact that protein vibrate due to either electrostatic force (potential) or due to external stimuli. In a paper (12) the authors suggested that the vibration characteristics of protein depend on the magnitudes of molecular weights of the concerned protein. It was also held by the authors (12) that more the molecular weights of protein is, the less is the number of vibration frequency. On the other hand, less the value of molecular weight of protein is, the more is the number of frequency. Thus the vibration pattern of proteins may be described as
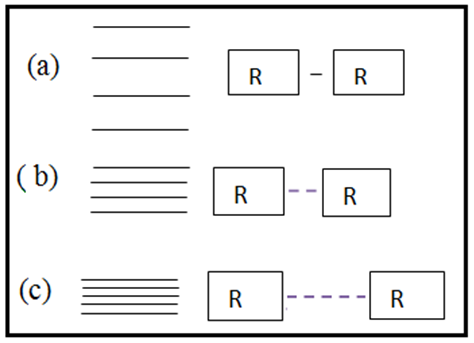 | Figure 1. Vibration energy spacing of protein molecules |
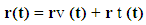 Where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation
Where rv (t) stands for the vibration component about the equilibrium position with the host molecule and rt (t) stands for transient component about the equilibrium position at time t. The electrostatic force required for this purpose may be deduced from the relation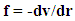 Which means force is nothing but rate of change of potential
Which means force is nothing but rate of change of potential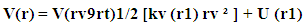 | (1) |
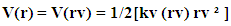 | (2) |
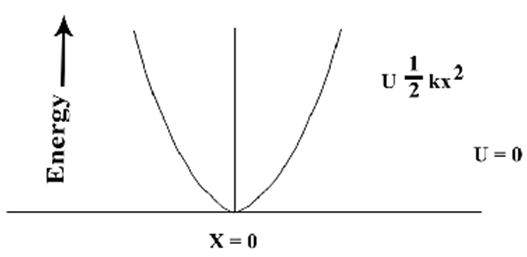 | Figure 2. Oscillation of vibrating particle |
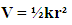 The one dimensional Schrodinger’s Equation
The one dimensional Schrodinger’s Equation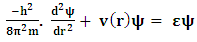 | (3) |
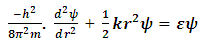 | (4) |
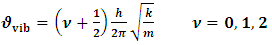 | (5) |
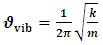 | (6) |
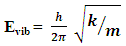 | (7) |
4. Results and Discussions
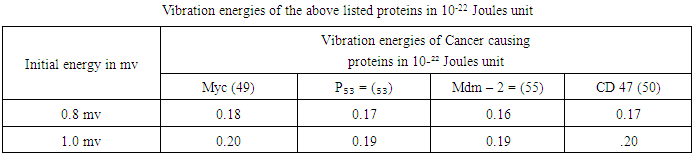
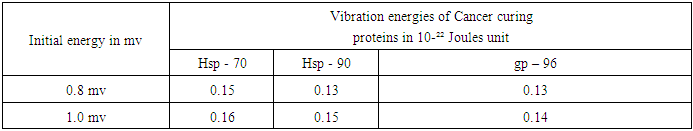
- Since a good number of proteins are responsible for the cause and cure of cancers, let us identify some of the proteins causing cancer as well as functioning as immune and therapeutic agents like
|
|
|
|
|
|
5. Conclusions
- Our analytical approach is found to be in parity with the experimental studies of the scholars with respect to the role of Hsps in many aspects of cancer / tumor immunity and therapeutic responses. Our studies may be essential in interpreting the information on how Hsp regulation is subverted in cancer and how Hsp intervenes the molecular events which take part in tumor growth, invasiveness and metastasis etc though at present it is hard to set-up adequate experimental arrangement at Tripura. Scholars are of the opinion that Hsps possessing significant properties may open the door for their inclusion in the next generation of vaccines to target DC. In this anticipation the scholars have categorized three important properties of Hsp which include (3) as (i) Hsps are natural adjuvant.(ii) Hsps are able to deliver multiple antigens which are capable to induce adaptive immune responses against pathogens and effective cancer.(iii) Data concerning different studies show that Hsps are safe constituents of existing vaccines.Similarly, Hsp 70 derived particularly from Bacopa Monnieri (B.M) and producing recombinant antigens may be utilized to develop multi epitomic vaccines. This means B.M induced Hsp 70 is also able to modulate the immune responses of cancer causing protein and protein receptors. In conclusion, it is our expectation that our studies many also stand in good stead in explaining immune response as well as therapeutic aspects of cancer / tumor – a devastating disease of the day.
ACKNOWLEDGEMENTS
- The author is thankful to Dr. U.C. De, Dept. of Chemistry, Tripura University India for helpful discussion. The author is also thankful to Miss. Joysree Sutradhar, for undertaking the Computer works.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML