-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2016; 6(3): 27-43
doi:10.5923/j.biophysics.20160603.01

On the Mechanisms of Wound Healing by Magnetic Therapy: The Working Principle of Therapeutic Magnetic Resonance
Larissa Brizhik1, Letizia Ferroni2, Chiara Gardin2, Enrico Fermi3
1Bogolyubov Institute for Theoretical Physics, Kyiv, Ukraine
2Department of Biomedical Sciences, University of Padova, Padova, Italy
3Thereson, Research & Development, Milan, Italy
Correspondence to: Larissa Brizhik, Bogolyubov Institute for Theoretical Physics, Kyiv, Ukraine.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Magnetic resonance therapy is innovative magnetic based strategy that, in these years, has received significant attention thanks to its positive medical effects. Clinical studies have shown that application of this therapy significantly accelerates wound healing and, in particular, healing of the diabetic foot disease. The aim of this paper is to formulate the working principle of this therapy. We analyse experimental data on biological effects produced by magnetic fields. Based on this, we show that there is a hierarchy of the possible physical mechanisms, which can produce such effects. We suggest that the mutual interplay between these mechanisms can lead to a synergetic outcome, which can affect the physiological state of the organism. Taking into account the non-thermal character of the magnetic therapy and experimental data on the tight connection between various diseases (including the wound processes) and changes in the redox processes, we study theoretically effects of magnetic fields on soliton mediated charge transport during the redox processes. We show that electrons bound in nonlinear soliton states in macromolecules, are sensitive to weak magnetic fields and change their dynamical properties. These changes affect charge transport and can facilitate redox processes. We suggest that enhancement of the redox processes launches the chain of biochemical and physiological changes which can stimulate the wound healing and healing of the organism in general. These non-thermal resonant mechanisms of the biological effects of magnetic fields, analysed in the paper, are summarized as the working principle of the magnetic resonance therapy. We support our approach by some biological and histological data (both in vitro and in vivo) and by some clinical data. The knowledge of this working principle can provide information useful for optimizing magnetic therapy treatment protocols, and can be used as the guidelines for a further, pivotal clinical investigations related to therapies based on magnetic fields.
Keywords: Wound healing, Magnetic resonance therapy, Soliton, Charge transport, Cyclotron resonance, Biological Effects of pulsating electro-magnetic field
Cite this paper: Larissa Brizhik, Letizia Ferroni, Chiara Gardin, Enrico Fermi, On the Mechanisms of Wound Healing by Magnetic Therapy: The Working Principle of Therapeutic Magnetic Resonance, International Journal of Biophysics , Vol. 6 No. 3, 2016, pp. 27-43. doi: 10.5923/j.biophysics.20160603.01.
Article Outline
1. Introduction
- The increasing need in non-invasive and non-chemical therapies as alternative to the drug-based medicine stimulates the research to find new resources. Lots of attention in these years has been related to the positive biological effects, caused by Electric and Magnetic Fields (EMFs) that are well established experimentally. The recent review of some of the biological effects, caused by EMFs of weak intensity in the broad interval of frequencies and intensities, can be found in Brizhik et al. [1]. Especially promising from the point of view of medical applications are low frequency (LF) and extremely low frequency (ELF) fields which are widely used in various therapies to treat broad class of diseases. In particular, one of such medical applications is based on the Therapeutic Magnetic Resonance TMR®, which turned out to show positive results in wound healing, and, in particular, in treatment of diabetic foot disease and vascular ulcers. The method consists of exposing patients to low intensity Pulsating Electro-Magnetic Fields (PEMFs), at specific patented protected shapes and low frequencies of pulses [2]. Successful application of this therapy and further improvement of its efficiency require understanding of the responsible physical mechanism(s) and knowledge of its working principle, which is the aim of the present paper. To advance in understanding of the relation between the biological structure, action of the magnetic field and reaction of the tissue and the whole organism to it, we organize our paper as follows.Below we give the brief review of the experimental data showing the biological effects of magnetic fields, describe the magnetic therapy method, summarize the main aspects of the processes of wound healing, provide the biological and clinical data of the results of this therapy, and, finally, develop the physical mechanisms, which can explain the action of this therapy. In an organism, which is highly nonlinear open system, these mechanisms are complementary, act simultaneously (at different time scales) and synergetically, although at different stages of the disease one or another can prevail. In Conclusions, summarizing these mechanisms, we formulate the working principle of the magnetic therapy.
2. Magnetic Therapy
- In his Section we briefly describe the magnetic therapy and its applications for wound healing. Some results of biological and medical study will be reported here as well.
2.1. The Experimental Background of Magnetic Therapy
- Electric and magnetic fields often occur together, nevertheless, namely magnetic field (MF) can be responsible for the biological effects, because MF is not screened by the skin, is not absorbed by cell cytoplasm, and can easily penetrate deep into biological tissues. Low-intensity LF and ELF electromagnetic fields are very promising from the point of view of their applications in medicine. The allostatic load on the organism exposed to such fields, is much less than in the case of high intensity and high frequency fields, even when we deal with the resonant mechanisms of the biological effects [3]. Exposed to LF or ELF low intensity MF, a cell (organ, system, and/or organism) can adapt without drastic consequences and a transient perturbation can be followed by an adjustment of the system via the normal homeostatic machinery of the cells. In such a way the system is not moved far from its quasi-stationary state and can adapt to new conditions in a more natural way. Such adaptation is accompanied by the tendency to restore the healthy state.Finally, it needs to be emphasized that no major, clinically important, side effects with the use of LF low-intensity MFs in numerous studies have been reported so far. The healthy system can even not respond to weak external stimuli at certain conditions. In terms of the theory of complex systems the ‘healthy’ and ‘ill’ states of an organism can be classified as two different attractors of the system in the multi-parameter phase space. These attractors correspond to quasi-stationary states, which have different free energies separated by the potential barrier. The transition between the two states requires the energy to overcome this potential barrier [3]. This is one of the reasons why the process of healing often occurs through the exacerbation phase (intensification of the disease) at the early stage of the treatment.It is worth to recall that the biological effects, caused by MFs, are manifested on all levels of the hierarchy of the living matter, in particular, on the levels of macromolecules, cells, organs and systems as well as the whole organism. The biological impact of the MF results from all biological pathways and processes, such as energy storage and transfer, charge (matter) transport, information transfer and exchange. Below we briefly describe some reliably established facts of the biological effects caused by MFs which, in our opinion, are important for their medical implementations.It is clear that a system can be sensitive to MF if it possesses charge and/or magnetic momentum (spin). Therefore, the primary biological ‘sensors’ of external MFs can be electrons, protons and ions, which are involved in all metabolic processes in living systems. These particles expire in a magnetic field
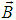 the Lorentz force
the Lorentz force 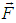 proportional to their charge, q, and velocity,
proportional to their charge, q, and velocity, 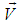 :
: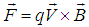 | (1) |
2.2. Using Magnetic Fields in Wound Healing
- Treatment of chronic wounds by means of EMFs [22] has demonstrated positive results. Moreover, such therapies turned out to be useful for treatment of patients with painful diabetic polyneuropathy (low sensation of nerves caused by uncontrolled blood glucose levels) [23-25]. Such therapies alone or in combination with existing clinical protocols can accelerate diabetic wound healing.There are several factors that influence wound healing in a diabetic patient. These include high blood glucose levels, poor blood circulation, high risk of infections and some other. In particular, an elevated blood sugar level leads to stiffness of the arteries and narrowing of the blood vessels. The effects of this are far-reaching and include the origin of wounds as well as an increase of risk factors for proper wound healing. Narrowed blood vessels lead to decreased blood flow and oxygen to a wound. An elevated blood sugar level decreases the function of red blood cells that carry nutrients to the tissue. This lowers the efficiency of the white blood cells that fight infection. Without sufficient nutrients and oxygen, a wound heals slowly. Diabetes lowers the efficiency of the immune system, the body's defence system against infection. A high glucose level causes the immune cells to function ineffectively, which raises the risk of infection for the patient. Studies indicate that particular enzymes and hormones that the body produces in response to an elevated blood sugar are responsible for negatively impacting the immune system. With a poorly functioning immune system, diabetics are at a higher risk for developing an infection. Infection raises many health concerns and also slows the overall healing process. Left untreated, infection can heighten the risk of developing gangrene, sepsis or a bone infection like osteomyelitis. According to statistics, diabetes is the primary reason for limb amputation in many countries.To elucidate the physical mechanisms of the magnetic therapies for wound healing, we need to summarize the biological processes, involved in the wound healing. The process of wound healing can be conditionally separated into four stages (phases) which are [26]:1. Hemostasis phase: formation of platelet plug, formation of a stable fibrin clot. It is a process which causes bleeding to stop, and involves blood changing from a liquid to a gel.2. Inflammatory phase (substrate-preparation phase) which lasts 1-4 days. It involves inflammation process and migration of cells, such as platelets, neutrophils, lymphocytes, macrophages, endothelial progenitor cells (EPCs). Endothelial cells are thin flattened cells which line the inside surfaces of body cavities, blood vessels, and lymph vessels. EPCs circulate in the blood and can differentiate into endothelial cells. 3. Proliferation phase (collagen-building phase) which lasts 2-22 days. It involves cell proliferation, the extracellular matrix (ECM) synthesis, angiogenesis, re-epithelialization. It includes such cells as keratinocytes, endothelial cells, fibroblasts, macrophages, EPCs. Fibroblasts are cells that synthesize the extracellular matrix and collagen which play a critical role in wound healing.4. Remodeling phase (maturation) which lasts 6-12 months and involves wound closure and contraction. Cell types involved are myofibroblasts, macrophages etc.The impaired vascular supply associated with diabetes, leads to poor blood flow at the wound site impeding the optimal endogenous reparative response [27]. In addition, neovascularization (formation of new blood vessels) is critical for granulation tissue formation and tissue regeneration in wound healing [28]. The impaired angiogenic response that occurs in diabetes mellitus leads to hypoxia at the wound site (lack of adequate oxygen supply). Temporary hypoxia is requisite for normal wound healing. In the non-diabetic situation, hypoxia leads to activation of the transcription factor complex HIF-1alpha (Hypoxia inducible factor-1alpha), which leads to transcription of multiple genes required for successful wound healing. With diabetes, hyperglycaemia affects the stability and activation of HIF-1alpha. This suppresses platelet-derived growth factor, vascular endothelial growth factor and transforming growth factor-beta, which are required for angiogenesis, in vitro and in vivo wound healing.
2.3. Cellular Biology Study of the Effects of TMR®
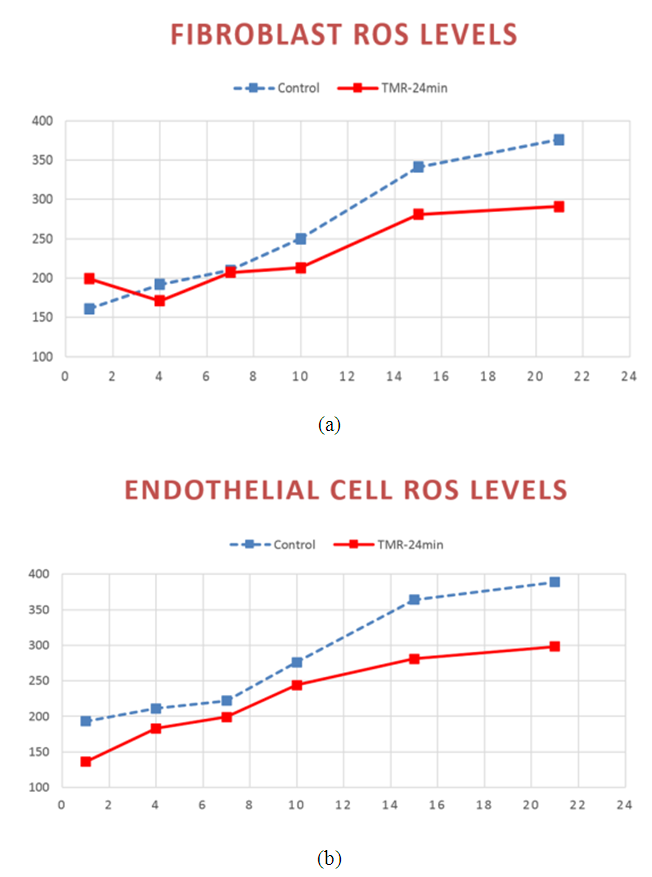
- An extensive cellular biology study has been performed by Ferroni et al [29] to ascertain the effects of TMR® stimulation on cell behavior. In particular, it has been demonstrated that PEMFs influence mitochondrial function, as it follows from Reactive Oxygen Species (ROS) measurements. ROS are chemically reactive molecules containing oxygen, such as oxygen ions and peroxides, formed as a natural byproduct of the normal metabolism of oxygen and have important role in cell signaling and homeostasis. Under conditions of oxidative stress the cell accumulates ROS. When the culture cells, both fibroblasts and endothelial cells, are treated with magnetic therapy, a well-defined decrease of ROS production is revealed, as it is shown in Figure 1.
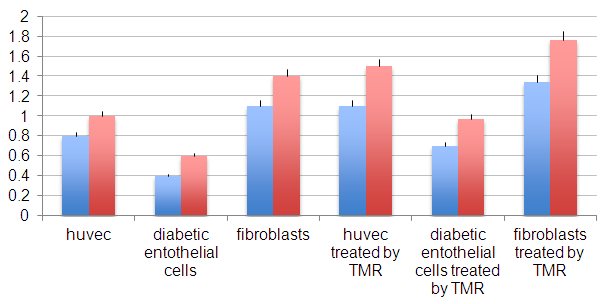 | Figure 2. Proliferation rate of endothelial cells (huvec), diabetic derived endothelial cells and fibroblasts control and treated by TMR® after 14 days (blue bars) and 21 days (red bars) |
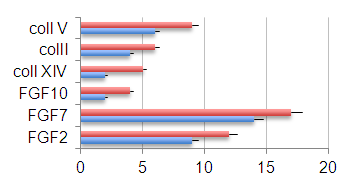 | Figure 3. Amount of extracellular matrix proteins involved in tissue repair process in control tissues (blue bars) and TMR-treated tissues (red bars) |
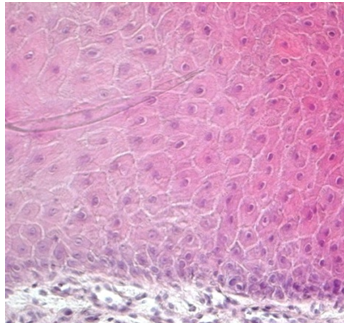 | Figure 4. The multilayer of keratinocytes growths onto a 3D artificial dermis previously treated with TMR® |
2.4. Clinical Data on Application of TMR® Therapy
- The clinical experience in the application of TMR® therapy to diabetic foot ulcer treatment began in 2012. It includes several studies [2]:1. A study on surgical patients, conducted by Prof. Piaggesi (Cisanello Hospital, Pisa, Italy) in January - March 2012 with the 12 months follow-up.2. A multi-center exploratory clinical study, conducted by three Italian clinical centers, in September 2012 – April 2014.3. A bioptic «in vivo» study (a part of the above study) conducted in December 2013 - March 2014, analyzed by University of Padua (Italy).An independent study on surgical patients conducted by A. Piaggesi [2] has shown statistically significant evidence of wound healing acceleration. In particular, the 90% healing rate at 6 months in the group treated with TMR® and 30% in group with standard treatment only (statistically significant; p<0.05) have been reported. Healing time in the group treated with TMR® was 84.46±54.38 days versus 148.54 ± 78.96 days in the control group (statistically significant; p<0.01). At the end of a 12-month follow-up period all the patients in the treated group were healed, while in control group three patients were still ulcerated.
3. The Main Mechanisms of Magnetic Resonance Therapy
- In this section we discuss the physical mechanisms which can be responsible for the wound healing under the exposure to PMFs. In the Introduction we have indicated that weak PEMFs can modify some biological processes. Taking into account the complexity of the biological process of wound healing, it becomes evident that the physical mechanism responsible for the impact of magnetic therapy is formed by a complex processes that cover several physical phenomena and biological processes on various levels of system organization. To be more specific in the present study, we will refer to the impact of MFs to the processes involved in wound healing. Of course, some of these processes and corresponding physical mechanisms are valid not only for wound healing, but are rather general and, therefore, can constitute the physical mechanism of magnetic therapy in general.
3.1. Cyclotron Resonance
- It has been shown that PEMFs induce cellular transcription [30] and that EMFs can regulate lymphocyte proliferation in vitro and in vivo experiments [6]. These effects can take place through the calcium channel activation and modified intracellular Ca2+ levels in the presence of MF.The basis of several important biological processes, including wound healing, involves ion-selective channels which enable the specific permeation of ions through cell membranes. Thus, Ca2+ ions in mitochondria are directly involved in the metabolism of cells. Voltage-dependent calcium channels are a significant part of the functioning not only of skeletal and smooth muscles, bones (osteoblasts), myocytes, dendrites and dendritic spines of cortical neurons, but also of the brain and peripheral nervous system. Ligand-gated calcium channels are located in plasma membrane. The functioning of both types of Ca2+ channels is reflected on the processes of wound healing. It has been proven experimentally that the intracellular Ca2+ ions concentration increases with exposure to ELF magnetic field, partly due to an increase in the number of activated Ca2+ channels. Therefore, one of the possible physical mechanisms can be based on the effects of MFs on functioning of Ca2+ ions. In particular, there is a Lorentz force acting on ions as they move through trans-membrane ion channels in the presence of a MF.Channels are formed by proteins that span the lipid bilayer membrane of the cell. These proteins can change their conformation on application of a mechanical force or an electrical field such that the new conformation can result in an opening or closing of the channel. The magnitude of force required to effect such a change, has been estimated to be in the range of 0.2 - 0.4 pN [31]. For some channels, this mechanical force can be actuated by application of an electrical potential difference across the membrane. In this case assuming a membrane thickness about 5 nm and a charged center in the ion channel protein of approximately 2 elementary charges, the necessary force should be 1 pN on the charged center in the protein. Charged centers in the protein respond to the electric field within the membrane, thus acting as transducers of the force. Such channels are usually described as voltage-gated channels and are typically activated by trans-membrane potential differences in the order of 20 mV. In the presence of a MF, Lorentz force, defined in Eq. (1), acts on ions as they move through trans-membrane ion channels. Drift velocity of charges in the channel, V, is of the order of 3x10-2 m/s. According to [32], the Lorentz force strong enough to activate nearby voltage gated ion channels, can be achieved in ELF magnetic fields as weak as 100 microT. This can be compared with the calculations of St. Pierre et al. [33] according to which the Lorentz force of the magnitude of 1 pN requires the MF intensity B =3x108 microT and the forces generated due to the Lorentz force of the MF on the cation is small as compared with the forces required to activate an ion channel protein conformation change associated with the gating of the channel. These estimates are based on the pure classical mechanical models. Obviously, one can hardly expect the ballistic transport of ions through the trans-membrane channels. There is experimental evidence that ion transport occurs in the form of the travelling waves [34]. Secondly, the calculations of forces necessary for the conformational changes of proteins, based on classical mechanical models, can give only rough results. According to [35], a self-consistent account of interaction between the carried charges and elastic degree of freedom of macromolecules (so called electron-lattice interaction) shows that propagation of charges along alpha-helical proteins is significantly facilitated and leads to self-trapping of charges in nonlinear soliton states (this will be discussed below). In an alpha-helical protein soliton propagation is accompanied by a local increase of the protein radius [36, 37], which can lead to the effective increase of the ion channel radius (comp. opening of the channel). But even these classical estimates show that weak MFs can facilitate permeability of calcium channels. Indeed, it has been demonstrated experimentally that neuronal ion channels are sensitive to ELF weak electric field effects [38].Another possible mechanism can be related with the effects of MFs on the cardiovascular system [4]. These effects on the microcirculation and vasculature can be different depending on the initial state of the cardiovascular system and on medical history of the patient. Thus, it has been reported in McKay et al. [4] that 10 of 27 studies showed vasodilatory effect, increased blood flow or increased blood pressure; 3 studies showed a decrease in blood perfusion/pressure; 4 studies showed no effect; and in 10 studies MF triggered either vasodilation or vasoconstriction depending on the initial tone of the vessels.Functioning of the cardio-vascular system depends significant on the effectiveness of respiration and accompanying it redox processes [39]. These processes occur together with the charge transport in the so called Krebbs cycle, which involves the electron transport chain. Such a chain represents a series of macromolecules onto which electrons can be transferred via redox reactions, so that each compound plays the role of a donor for the 'preceding' molecule and acceptor for the 'succeeding' molecule. Some molecules in the electron transport chains, such as quinone or cytochrome cyt-c, possess relatively small molecular weight. They are highly soluble and can move relatively easily outside the mitochondrial membrane, carrying electron from a heavy donor to a heavy acceptor. Some other molecules in the electron transport chain, such as NADH-ubiquinone oxidoreductase, flavoproteids, cytochrome c-oxidase cyt-aa3 and cytochrome cyt-bc1 complexes are proteins with large molecular weight and thus they are practically fixed in the corresponding membrane. A significant fraction of these proteins is in the alpha-helical conformation, which can support the transport of electrons in the form of electrosolitons [35, 40]. Namely these electrosolitons (also called briefly solitons) are sensitive to the constant and PMFs.Three basic physical mechanisms of biological effects of MFs have been identified by Brizhik et al [1] depending on the hierarchy level of the matter organisation at which such effects takes place: molecular, supramolecular and system mechanisms. Molecular mechanism involves effects of MFs on ions, radicals, paramagnetic particles with electrons and spin, molecules, macromolecules. Supramolecular mechanism involves effects of MF on membranes, mitochondria, microcrystals, cell nuclei, cells etc. System mechanism is based on the synergetic effect of molecular and supramolecular effects and is manifested on the level of the biological system (endocrine, cardiovascular, nerve, etc.). The biological effects via this latter mechanism are more delayed in time, since they result from the primary effects of the first two mechanisms, which are processed with time by the corresponding system and by the whole organism.Electromagnetic field interaction with living tissues can be realized through these three main mechanisms, affecting energy transfer, charge (matter) transport, and information transport/exchange. Various cellular components, processes, and systems can be affected by EMF exposure. Although there are numerous studies and hypotheses that suggest that the primary sites of interaction are membranes, there are also numerous in vitro studies indicating that systems, including cell-free systems, can be responsive to EMFs [41]. In particular, ELF electromagnetic fields can affect cellular responses in vitro tests, causing immune cell activation [42].Dependence of the biological effects of oscillating MFs on the frequency indicates the resonant character of such effects. Among such resonant mechanisms the first to be mentioned, is the cyclotron resonance. In biological systems there are electrons, various ions and groups of ions, which are sensitive to the alternating MFs. Due to the Lorentz force, a charge q=Ze (an electron, Z=1, or an ion of the charge Ze) of the mass m in a static MF of the intensity B moves along the circular trajectory with the angular frequency:
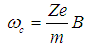 | (2) |
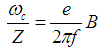 | (3) |
3.2. Soliton Mechanism
3.2.1. Molecular Solitons and Electrosolitons
- Another sensor of the external MF can be molecular solitons and electrosolitons, which we call below by the same term ‘soliton’ [35, 40]. We remind here that a molecular soliton (called also as Davydov’s soliton) is a nonlinear bound state of a molecular excitation like AMID-I excitation and local deformation of quasi-one-dimensional molecular chain, like, for instance, polypeptide chain in macromolecules. Similarly, an electrosoliton is a bound state of an electron and local deformation of the macromolecule. It has been shown theoretically that Davydov’s solitons provide storage and transfer of the ATP hydrolysis energy in cells [35, 40].According to Brizhik et al. [50], electrosolitons provide transport of electrons in certain steps of redox processes during respiration, as is summarized below. This transport takes place along the so-called electron transport chain [39, 51], which represents a series of biological molecules that transfer electrons from one to another via redox reactions, so that each compound plays the role of a donor for a molecule 'on the left' and acceptor for the molecule 'on the right'. In eukaryotes the electron transport chain is located in inner mitochondrial membrane, where oxidative phosphorylation with ATP synthase takes place [39, 51]. Some molecules in the electron transport chains, like quinone or cytochrome cyt-c, have relatively small molecular mass. They are highly soluble and can move relatively easy outside the mitochondrial membrane, carrying an electron from a heavy donor to a heavy acceptor. In theoretical studies such electron transport systems are modeled as complexes which include a donor molecule weakly bound to a bridge molecule, which in its turn is weakly bound to an acceptor molecule. The bridge itself can be modeled as some potential barrier through which the electron tunneling takes place [51]. In some other studies the bridge is modeled as a molecule with super-exchange electron interaction taken into account. It has been shown that properties of electrons in these systems differ little from properties of free electrons. Therefore, their cyclotron resonance frequency is close to the frequency determined in Eq. (2) setting Z=1.Some other molecules in the electron transport chain, such as NADH-ubiquinone oxidoreductase, flavoproteids, cytochrome c-oxidase cyt-aa3 and cytochrome cyt-bc1 complex are proteins with large molecular weight, and, thus, they are practically fixed in the corresponding membrane [39]. Qualitatively and quantitatively different situation takes place for electrons, when they are transported through these proteins of large molecular mass. First of all, a significant part of such proteins is in alpha-helical conformation, which is stabilized by relatively weak hydrogen bonds between every fourth peptide group (a group of atoms H-N-C=O), so that along the helix there are three hydrogen-bounded polypeptide chains. The softness of hydrogen bonds and quasi-one-dimensional structure of polypeptide chains suggests a possible significant role of the electron-lattice interaction in them. Indeed, it has been shown [35] that this electron-lattice interaction is relatively strong and that it results in a self-trapping of electrons: electrons, transferred into a protein from a donor molecule, create a local deformation of the protein. Such deformation acts as a potential well, which attracts an electron. As a result, a bound state of an electron and lattice deformation is formed. This state is described by the system of coupled nonlinear equations for the electron wave-function,
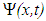 , and lattice deformation,
, and lattice deformation, 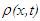 :
: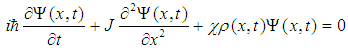 | (4) |
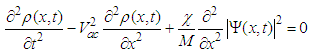 | (5) |
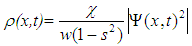 | (6) |
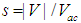 . It is worth to recall that the electron wave function is normalized to 1, since square of its modulus determines electron probability.Substituting this solution into Eq. (4), we derive the so-called nonlinear Schroedinger equation:
. It is worth to recall that the electron wave function is normalized to 1, since square of its modulus determines electron probability.Substituting this solution into Eq. (4), we derive the so-called nonlinear Schroedinger equation: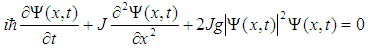 | (7) |
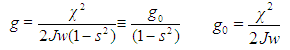 | (8) |
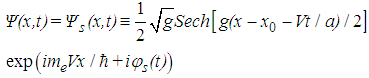 | (9) |
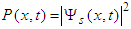 , and, according to the relation (6), the chain deformation, are localized in space with the width of the localization
, and, according to the relation (6), the chain deformation, are localized in space with the width of the localization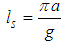 | (10) |
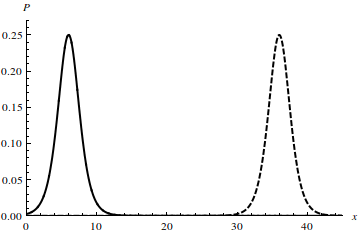 | Figure 5. Probability of electron localization, P, in a soliton state along the chain axis x at the parameter values g = 1, x0 = 5 at time moments t = 0 (solid line) and t = 30a/V (dashed line) |
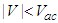 , and is exceptionally stable, able to propagate on macroscopic distances due to the extremely low dissipation of energy and the nonlinear nature of it formation. The energetical and dynamical stability of nonlinear soliton states is due to the compensation between the dispersive effects of wave processes and nonlinearity of the system, i.e., electron-lattice interaction. In the result of this interplay solitons propagate along macromolecules with very low energy dissipation, which explains high efficiency of the bioenergetics and DNA transcription. If the closure of the double chain after the RNA polymerase has passed, is done in a non-coordinated way, it will generate a substantial quantity of random motion and, thus, of thermal energy. If, instead, this happens after the passing of a nonlinear excitation, very little thermal energy is generated during the system recovery of the ground state.In view of the binding of an electron with the lattice deformation, the effective mass of a soliton is bigger than the mass of a free electron, me:
, and is exceptionally stable, able to propagate on macroscopic distances due to the extremely low dissipation of energy and the nonlinear nature of it formation. The energetical and dynamical stability of nonlinear soliton states is due to the compensation between the dispersive effects of wave processes and nonlinearity of the system, i.e., electron-lattice interaction. In the result of this interplay solitons propagate along macromolecules with very low energy dissipation, which explains high efficiency of the bioenergetics and DNA transcription. If the closure of the double chain after the RNA polymerase has passed, is done in a non-coordinated way, it will generate a substantial quantity of random motion and, thus, of thermal energy. If, instead, this happens after the passing of a nonlinear excitation, very little thermal energy is generated during the system recovery of the ground state.In view of the binding of an electron with the lattice deformation, the effective mass of a soliton is bigger than the mass of a free electron, me: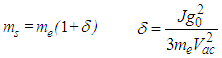 | (11) |
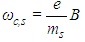 | (12) |
3.2.2. Effect of Magnetic Field on Soliton Dynamics
- A more rigorous study of the dynamics of an electrosoliton in a MF requires extension of the one-dimensional model to the 3-dimensional space, when all functions depend on three spatial coordinates:
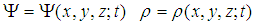 | (13) |
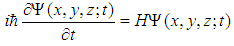 | (14) |
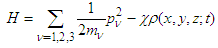 | (15) |
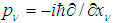 is the corresponding component of the momentum operator of an electron, and 1/mv is the tensor of the electron effective mass.Deformation of the polypeptide chain oriented along x-direction is determined by the equation which is the generalization of Eq. (5) to a 3D space:
is the corresponding component of the momentum operator of an electron, and 1/mv is the tensor of the electron effective mass.Deformation of the polypeptide chain oriented along x-direction is determined by the equation which is the generalization of Eq. (5) to a 3D space: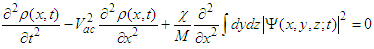 | (16) |
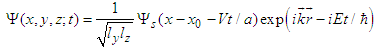 | (17) |
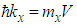 , and
, and 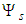 is defined in Eq. (8).In the presence of the external MF,
is defined in Eq. (8).In the presence of the external MF, 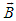 , dynamics of an electron is governed by the system of equations (13), (16), in which the Hamiltonian includes the vector-potential of the MF,
, dynamics of an electron is governed by the system of equations (13), (16), in which the Hamiltonian includes the vector-potential of the MF, 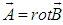 ,
,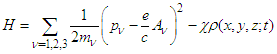 | (18) |
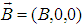 , and choose the vector-potential in the form
, and choose the vector-potential in the form 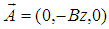 . The Hamiltonian (17) can be represented in the form of the sum of the soliton part, Hs, and transversal part, Ht :
. The Hamiltonian (17) can be represented in the form of the sum of the soliton part, Hs, and transversal part, Ht :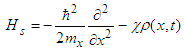 | (19) |
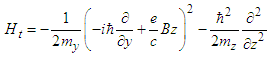 | (20) |
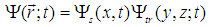 | (21) |
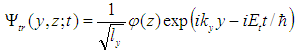 | (22) |
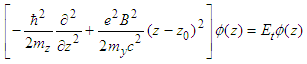 | (23) |
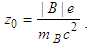 | (24) |
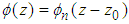 , with the energy:
, with the energy: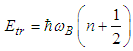 | (25) |
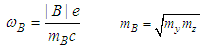 | (26) |
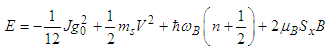 | (27) |
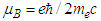 is Bohr magneton and
is Bohr magneton and 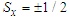 is the projection of the electron spin on the direction of the MF.The energy level (25) is degenerate, the degree of degeneracy is determined by the number of the possible values of the momentum component ky at which the equilibrium position of the electron z0 is located inside the macromolecule.2. Soliton in a transverse magnetic fieldLet us now consider the case when the MF is perpendicular to the chain, for instance, is oriented along z-axis
is the projection of the electron spin on the direction of the MF.The energy level (25) is degenerate, the degree of degeneracy is determined by the number of the possible values of the momentum component ky at which the equilibrium position of the electron z0 is located inside the macromolecule.2. Soliton in a transverse magnetic fieldLet us now consider the case when the MF is perpendicular to the chain, for instance, is oriented along z-axis 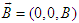 . In this case we can choose the vector-potential in the form
. In this case we can choose the vector-potential in the form 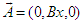 .Then the Hamiltonian (17) can be represented in the form
.Then the Hamiltonian (17) can be represented in the form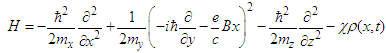 | (28) |
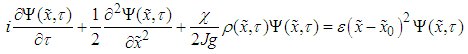 | (29) |
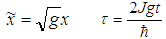 | (30) |
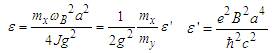 | (31) |
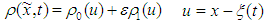 | (32) |
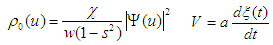 | (33) |
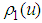 :
: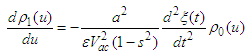 | (34) |
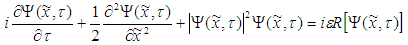 | (35) |
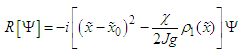 | (36) |
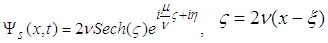 | (37) |
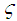 and
and 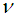 are the parameters which determine the width and amplitude of the soliton and, and
are the parameters which determine the width and amplitude of the soliton and, and 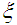 is the center of mass coordinate of the soliton. All these parameters weakly depend on time:
is the center of mass coordinate of the soliton. All these parameters weakly depend on time: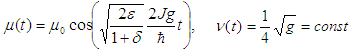 | (38) |
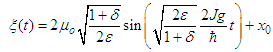 | (39) |
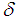 is defined in Eq. (10) and
is defined in Eq. (10) and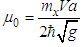 | (40) |
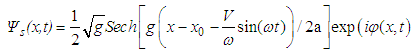 | (41) |
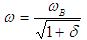 | (42) |
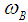 is determined by the expression (26).Therefore, soliton wave function in the transverse MF is given by the expression (41). It describes propagation of the localized wave package with the envelope which coincides with the envelope of a free soliton, but whose velocity of propagation is oscillating in time with frequency of oscillations which depends on the MF:
is determined by the expression (26).Therefore, soliton wave function in the transverse MF is given by the expression (41). It describes propagation of the localized wave package with the envelope which coincides with the envelope of a free soliton, but whose velocity of propagation is oscillating in time with frequency of oscillations which depends on the MF: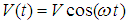 | (43) |
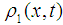 which also is the oscillating function of time. This deformation will excite additional vibrational modes in the polypeptide chain and can change its conformation, which can be reflected on the conformation-function relation. Such structural changes can be critical for many processes, from cellular communication through membrane ion channels to oxygen uptake and delivery by hemoglobin. Indeed, the long-range protein vibrational modes have been optically registered in [53]. In particular, binding of the so-called T cells which are known to be the key modulators of inflammation, can be affected by the MF. It has been shown that 0.1 mT, 60 Hz EMFs induce a 20% mean-increase in anti-CD3 binding to T cell receptors (TcRs) of Jurkat cells, a T lymphocyte cell line [6]. It also has been shown there that 60 Hz sinusoidal EMFs and a commercial bone healing EMF modulate signal transduction pathways that regulate lymphocyte proliferation and that are normally triggered by activating the Jurkat TcR. Similar EMF effects have been shown in human peripheral blood lymphocytes (hPBLs), exposed to EMFs in culture and in rat PBLs, when donor animals are exposed to a bone healing field (21 days, 4 hr/day). Thus, we see that the MF affects the electrosoliton transport, and, therefore, it can affect the redox processes. Indeed, the electromagnetic induction of protection against oxidative stress has been demonstrated experimentally [54].
which also is the oscillating function of time. This deformation will excite additional vibrational modes in the polypeptide chain and can change its conformation, which can be reflected on the conformation-function relation. Such structural changes can be critical for many processes, from cellular communication through membrane ion channels to oxygen uptake and delivery by hemoglobin. Indeed, the long-range protein vibrational modes have been optically registered in [53]. In particular, binding of the so-called T cells which are known to be the key modulators of inflammation, can be affected by the MF. It has been shown that 0.1 mT, 60 Hz EMFs induce a 20% mean-increase in anti-CD3 binding to T cell receptors (TcRs) of Jurkat cells, a T lymphocyte cell line [6]. It also has been shown there that 60 Hz sinusoidal EMFs and a commercial bone healing EMF modulate signal transduction pathways that regulate lymphocyte proliferation and that are normally triggered by activating the Jurkat TcR. Similar EMF effects have been shown in human peripheral blood lymphocytes (hPBLs), exposed to EMFs in culture and in rat PBLs, when donor animals are exposed to a bone healing field (21 days, 4 hr/day). Thus, we see that the MF affects the electrosoliton transport, and, therefore, it can affect the redox processes. Indeed, the electromagnetic induction of protection against oxidative stress has been demonstrated experimentally [54].4. Discussion
- Below we describe the data, which in our opinion provide the experimental support of the mechanisms developed in the previous section. They are based on the clinical experience (study on surgical patients) [2] which shows statistically significant evidence of healing process acceleration.In vitro tests both on healthy and diabetic tissue under the exposure to TMR® show not only an acceleration of fibroblast and endothelial cells growth after the exposure, but also the reduction of oxidative stress condition both in fibroblast and endothelial cells. Clinical experience of TMR® usage in a double-blind exploratory clinical study results in a dramatic reduction of lesion surface (as compared to conventional treatment) and lesion volume.These data show the direct correlation between the decrease of the oxidative stress and wound healing. The oxidative stress, in its turn, results from the inhibition of the redox processes. As it follows from our theoretical study in the previous section, the MF can enhance/restore charge transport and, thus, improve the redox processes, reducing oxidative stress.In the previous section we have indicated, that soliton mechanism describes the storage and transfer of the energy of ATP hydrolysis and have shown sensitivity of solitons to MF. Indeed, it has been shown experimentally that ATP synthesis can be induced by PEF [55] and that MF affects enzymatic ATP synthesis [56].In inflammation massive infiltration of T-lymphocytes, neutrophils and macrophages into the damaged tissue takes place [57]. Based on the presence of A2A adenosine receptors in human neutrophils, it has been suggested that adenosine plays an important role in modulating immune and inflammatory processes. Therefore, activation of A2A receptors by PEMFs may have a relevant therapeutic effect [58, 59]. Neutrophils are the most abundant white cells in the peripheral blood and are usually the first cells to arrive at an injured or infected site. Adenosine, interacting with specific receptors on the surface of neutrophils, is an endogenous anti-inflammatory agent [18]. The activation of A2A receptors in human neutrophils affects the immune response in auto-immune and neurodegenerative diseases and decreases inflammatory reactions [60]. Exposure to PEMFs can suppress the extravascular edema during early inflammation [61]. It has also been demonstrated that the complete healing of wounds depends on the presence of A2A adenosine receptor agonists [62]. It has been reported that PEMFs mediate positive effects on a wound healing, controlling the proliferation of inflammatory lymphocytes and resulting in beneficial effects on inflammatory disease [63]. Exposure to PEMF can trigger a more complex biologic response such as cell proliferation as it is evident from some clinical results [64, 65]. It can induce an increase in the proliferation of human articular chondrocytes suggesting an important role also in cartilagine repair [66].In biological systems there is a whole set of signaling mechanisms, known as transduction pathways, which involve various biochemical reactions, some of which take place with participation of ionic radicals [39]. Cells as no other known system can convert one type of stimulus into another, using chains of biochemical reactions involving enzymes. Enzymes are activated by specific molecules, called messengers, for instance, the cellular calcium ion, Ca2+. Therefore, they can be affected by oscillating MFs. The experimental evidence of this has been given in the previous section. It has been reported that through the ion cyclotron resonances the MF can be useful in the regenerative medicine [67]. Oscillating MF effects human epithelial cell differentiation through the ion cyclotron resonance [68, 69]. Calcium and potassium ions can be specifically activated by the MF through this effect, which enhances their transport through membrane ion channels, thereby altering signaling mechanisms and cellular function [70]. These signals are mediated in cells by the cytoplasm, in which water is one of the main components.Another important biological process, sensitive to MF, is DNA transcription by polymerase. Like ATP synthesis, catalysis of DNA transcription involves metal ions (Mg, Zn) and also shows significant magnetic isotope effect for magnetic ions 199Hg, 25Mg, 67Zn, 43Ca [46, 47]. The transcription is initiated by the mechanical unwinding of DNA at the promoter site. The RNA polymerase (RNAP) can be viewed as a torque wrench, which opens a ‘transcription bubble’ of a size of about 15–20 base pairs, that travels along the DNA as a solitary wave keeping the size of the open region almost constant, opening the double chain in front of the RNAP and closing the chain behind it. It is assumed that the transcription bubble travels along the chain in the shape of the soliton used by RNAP to access the base sequence [48]. This process can be altered by pathological conditions. The TMR® can help functioning of normal cells reactivating the physiological processes. In particular, as we have discussed above, the interaction of the weak MF of the TMR® with biological tissue, can be related to the mechanical action produced by the field itself on the cells in the form of the Lorentz force Eq. (1). Also a rotational torque acts on DNA topology, helping launching soliton, and so stimulating cell replication. Indeed, it has been shown experimentally that pulsating EMFs can induce cellular transcription [30]. The hypothesis that transcription responses depend on pulse characteristics was evaluated by using two pulses in clinical use, the repetitive single pulse and the repetitive pulse train. These pulses produced different results from each other and from controls. The single pulse increased the specific activity of messenger RNA after 15 and 45 minutes of exposure.The mechanism of magnetic isotope effect is connected with the interaction of the unpaired electron of cation-radical Mg+ with the nuclei, which results in the change of the electron spin of the pair: spin conversion of the pair from a singlet to a triplet state takes place. Such change of the spin opens a new reaction channel in a radical pair. It has been shown that enzymes with magnetic ions 25Mg2+ can be activated by MF, while enzymes with a nonmagnetic 24Mg2+ are inhibited [71]. In a similar way external MF can control such reactivity.The soliton mechanism of the redox processes in respiration, described briefly previously, is supported by some experimental data. In particular, it has been shown that weak magnetic RF and SMFs increase rate of hemoglobin deoxygenation in a cell [46]. Catalysis of oxidation of nicotinamide adenine dinucleotide (NADH), which participates in the redox processes, by molecular oxygen, is performed by peroxidase enzyme. This oxidation is an oscillating reaction with the period of oscillations approximately 100 s, during which concentrations of NADH and O2 oscillate. It has been shown that these reactions depend on the period of oscillations and intensity of the MF in the interval 1000-4000 Gs [72].It is well known that free radicals are constantly formed in the body during normal metabolic processes and are directly involved in inflammatory processes. When their formation is significantly increased, or protective mechanisms are compromised, a state of oxidative stress will result. If oxidative stress is persistent, it will lead to molecular damage and tissue injury. The antioxidative effect of the MF via the soliton mechanism, helps to reduce free radicals and consequently the oxidative damage, also increasing the antioxidative defence. Oxidative stress and apoptosis in relation to exposure to MF has been discussed above and also demonstrated experimentally in [73]. This can be one of the mechanisms responsible for the TMR® therapy as well.The treatment with specific frequencies of electromagnetic waves corresponding to some optimal regime to optimize the redox balance (rH2) and the acidity (pH) of body fluids to restore the cellular metabolism, has been reported in [71]. A significant improvement in the wound closure and bone fractures healing process, improvement of osteogenesis in osteoporosis have also been shown in [71, 74, 75], as well as in wound healing [76]. A significant decrease of ROS production, when the cultures of fibroblasts and endothelial cells were treated with TMR®, has been revealed experimentally (Figure 1). The time-dependent decrease of ROS productions, and, hence, of metabolic activity in the cells treated with the PEMFs, is also confirmed by Ferroni et al. [29].These data confirm the ability of TMR® to influence mitochondrial function, in particular, and the cellular energetics in the whole. These facts indirectly support our model as well. We remind here that fibroblasts are cells that synthesize the extracellular matrix and collagen and play a critical role in wound healing. Endothelial cells are thin flattened cells which line the inside surfaces of body cavities, blood and lymph vessels , and, thus, are also involved in wound healing.We can’t skip the subject of possible contradictions between the data obtained by various authors on the therapeutic effects of MFs and/or low reproducibility of such data. It is worth to recall, that living organisms possess complex structure and organization, which are determined by a huge number of factors (parameters). Due to this, biological systems can be very sensitive to some of these parameters and much less sensitive to others, which makes it difficult to reach a high reproducibility of the experiments with living organisms. Moreover, the biological impact of the external stimuli in general, and of MF in particular, can depend on the phase of the development of biological cells and their synchronization if any, ‘history’ of the system under study, etc. We have discussed this problem in the aspect of biological effects in our previous paper [1]. This is the case even at the lower level of living matter organization: the age and state of the cell can profoundly affect the EMF biological response [41]. The effects of MF exposure on microcirculation and microvasculature shown to be different [4]: approximately half of the cited there studies indicated a vasodilatory effect of MFs; the remaining half indicated that MFs could trigger either vasodilation or vasoconstriction depending on initial vessel tone. Indeed, perception and sensitivity to exposure not only depend on field parameters, including the field intensity, frequency, modulation, duration of the exposition, etc., but also can vary for different people, as it follows from numerous studies [11]. In humans, the biological response is dependent not only on exposure at particular MF strengths and frequency, but also on specific shape of MF pulses. The results on the use of magnetostimulation in treatment of patients with painful diabetic polyneuropathy are to a certain extent contradictory [23-25]. Thus, the positive effects of such treatments in reducing pain intensity, improving quality of life, and decreasing sleep disturbances, etc., have been reported [23, 24]. Other authors concluded that the genuine MF exposure had no advantage over sham exposure [25]. Comparison of these results is difficult because of differences between the MF parameters exposure duration, total exposure times and devices used to generate the MFs. The biological response occurs only within a specific amplitude and frequency range, being moderate or absent outside of this ‘window’. This can explain the contradicting findings in the studies using different exposure profiles by different authors.
5. Conclusions
- We have shown above that there are several physical mechanisms which cause biological effects of MFs, in general, and, in particular, can result in the positive impact of magnetic therapies, such as, for instance, magnetic resonance therapy, registered as TMR® therapy, when patients are exposed to low-intensity PEMFs at specific patented shapes and low frequencies of pulses. We have reported above “in vivo” confirmation of TMR® efficacy in acceleration of skin tissue regeneration in wound healing process of diabetic and non-diabetic patients, and, in particular in wound healing of the diabetic foot disease. To understand the working principle of this therapy, we analysed relevant to it biological effects produced by MFs. Based on these data, we have shown that there is a hierarchy of the possible physical mechanisms, which can produce such effects. These mechanisms act on different levels of the hierarchy of the organization of living organisms and at different time scales.Taking into account the non-thermal character of the magnetic therapy and experimental data on the tight connection between various diseases (including the wound processes) and changes in the redox processes, we have studies theoretically effects of magnetic fields on soliton mediated charge transport during the redox processes. We have shown that soliton mediated energy and charge transport during metabolism is sensitive to MFs, so that such fields can facilitate energy storage and transfer, and can enhance redox processes, which, in its turn, can stimulate the healing effect of the organism in general. We have shown that within the soliton mechanism of energy and charge transport, the MF can cause a hierarchy of changes from the primary effect on the dynamics of solitons, to the changes of the conformational states of macromolecules, to the effects on the rate of respiration, and, finally, to the effect on the whole metabolism of the organism.In conclusion, we summarize the above analysis of the biological effects of MFs as the three main mechanisms which can explain the interaction between magnetic therapies and healing processes, in particular, between Therapeutic Magnetic Resonance TMR® and biological tissue in wound healing process:1. Effect of MF on nonlinear excitations involved in the DNA transcription process (RNA polymerase). This effect is attained in the result of the local therapy.2. Increase of the effectiveness of the ATP hydrolysis energy storage and transfer in cells in the form of Davydov’s solitons. This effect is attained in the result of the local therapy and total body exposure.3. Increase of the effectiveness and synchronization of redox processes in the result of effects of MF on electrosolitons. This effect is attained in the result of the both local therapy and total body exposure.These mechanisms are essentially non-thermal and can be caused by low-intensity MFs, which in fact are used in magnetic therapies. The mutual interplay between these mechanisms can lead to a synergetic outcome (that can be delayed in time) which can affect the physiological state of the organism.According to biology-histopathology evidence and exploratory clinical investigation, TMR® therapy looks as a promising technique to treat early stage and advanced stage diabetic foot ulcers, significantly accelerating their healing process. The underlying mechanisms and biological effects of MFs on the processes involved in wound healing, have been summarized as the working principle called as ‘Brizhik-Fermi working principle of the TMR® therapy’ at the THERESON Scientific Meeting (Piacenza, Italy, June 06, 2014) and is shown schematically in Figure 6.
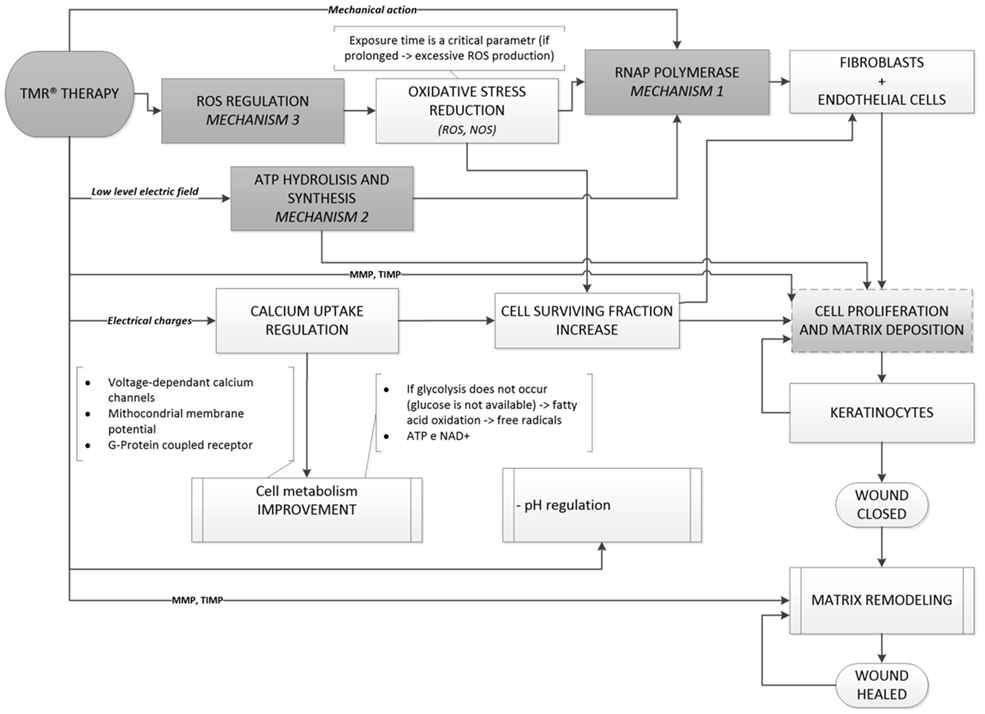 | Figure 6. The scheme of the Brizhik-Fermi working principle of the TMR® therapy |
ACKNOWLEDGEMENTS
- The authors acknowledge stimulating discussions with C. Simmi and D. Zanotti. This research was carried under the partial support from the Fundamental Research grant of the National Academy of Sciences of Ukraine.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML