Ataklti Abraha1, 2, AV Gholap2, Abebe Belay3
1Department of Physics, School of Natural and computational Sciences, Samara University, Samara, Ethiopia
2Department of Physics, College of Natural Science, Addis Ababa University, Addis Ababa, Ethiopia
3Department of Physics, School of Natural Sciences, Adama Science and Technology University, Adama, Ethiopia
Correspondence to: Ataklti Abraha, Department of Physics, School of Natural and computational Sciences, Samara University, Samara, Ethiopia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this paper UV-Vis spectroscopy was used to study the self-association, optical transition probabilities (molar decadeic absorption coefficient, integrated absorption cross-section, transition dipole moment and oscillator strength of the spectra) and thermodynamic properties of Neomycin sulfate in aqueous solution at room temperature (295K). The optical transition probabilities were calculated by integrated absorption coefficient techniques in the wave number range 27173.9 - 37650.6 cm-1. The dimerization constant of Neomycin Sulfate (1.062 105 M-1) was analyzed using dimer model by nonlinear curve fitting to the experimental values. In addition the thermodynamic parameters (Gibbs free energy, enthalpy and entropy) of dimerization reactions for the self -association of the compound were investigated using Vant’s Hoff equation at the temperature range (295 –312)K. Thus, the change of enthalpy calculated for Neomycin Sulfate is (3.765 ± 0.666) ΔH/kJ.mole-1. The values of change in enthalpy and entropy indicate that the hydrophobic interaction play the major role in the interaction between the molecules of Neomycin sulfate.
Keywords:
Neomycin Sulfate, Optical Transition Probabilities, UV-Vis spectroscopy, Thermodynamic Properties, Self-Association
Cite this paper: Ataklti Abraha, AV Gholap, Abebe Belay, Study Self-association, Optical Transition Properties and Thermodynamic Properties of Neomycin Sulfate Using UV-Visible Spectroscopy, International Journal of Biophysics , Vol. 6 No. 2, 2016, pp. 16-20. doi: 10.5923/j.biophysics.20160602.02.
1. Introduction
Neomycin Sulfate is a water soluble hydrophilic aminoglycoside antibiotic and calcium channel protein inhibitor that binds to prokaryotic ribosomes inhibiting translation, and is highly effective against Gram-positive and Gram-negative bacteria (Waksman, 1950; Xue et al., 2010). It is commonly used for the prevention of bacterial contamination of cell cultures and for treatment in various eye, skin and gastrointestinal infections (Zejc & Gorczyca, 2002; Patel, Dave, 2015; Darwhekar, Jain, Choudhary, 2012). Neomycin sulfate inhibits DNase I induced DNA degradation (Xue et al., 2010). Investigating the self-association, optical transition and thermodynamic properties of such compound are very useful in optics, biotechnology, medical imaging, medicine, drug delivery, structural materials (Sunil Kumar, Yousaf Khan, Verma, Chakarvarti, 2008). Determination of these properties are important to characterize and understanding the biological binding system, to know interaction strength in liquid solution and electron transition of the molecules, and interpretation of absorption spectral of the compound (Belay, 2013; MacRae, 1957; Milonni and Eberly, 1988). The knowledge of the optical properties may also applicable for experimental work (in absorption, emission and dispersion), and in characterize and stringent test of atomic and molecular structure calculations of the compound in theoretical work (Belay, 2013; Bayliss, 1950; MacRae, 1957; Milonni and Eberly, 1988). Different spectroscopic techniques were developed for investigating the optical transition properties of a compound. In this study, the simplest and more powerful spectroscopic technique in measuring the intensity of absorption light called integrated absorption technique were used using UV/Vis spectroscopy (Belay et al., 2010; Belay, 2013; Niazi et al., 2006). The technique is highly sensitive, rapid and easily implemented. Therefore, the objective of this study is to investigate the Self-association, optical transition probability and thermodynamic parameters of Neomycin Sulfate, since these properties are not yet investigated in this regard.
2. Materials and Methods
The Perkin-Elmer Lambda 19 UV-Vis Spectrophotometry with double monocromator and 1 cm quartz cuvette was used for the electronic absorption measurements of the investigated solution of Neomycin Sulfate at room temperature (293K). The analyzed spectra were obtained by subtracting the spectrum of pure solvent (water) from that of the solution containing Neomycin Sulfate.Neomycin Sulfate (NOMS, Fig. 1) was bought from Sigma-Aldrich and used for measurements without any further purification. The experimental method implemented to study of self-association of the compound was similar to the method applied by Belay (2012), for self and hetero association of caffeic acid. The self - association of Neomycin Sulfate was studied over the concentration range of (3.1 - 1.65)10-2M. The absorbance as a function of concentration has been measured at absorption maxima 304.8 nm to obtain the greatest accuracy of detection. For numerical analysis the dimer model equation were used by fitting to the experimental data by non-linear curve fitting based on Levenberg-Marquardt algorithm using origin 8 software. The molar extinction coefficients and equilibrium constant were used as searching parameters, in order to achieve minimum discrepancy between the experimental data and theoretical model.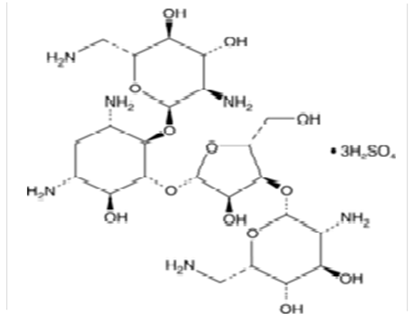 | Figure 1. Chemical Structure of Neomycin Sulfate |
The optical transition probabilities (transition dipole moment, oscillator strength and integrated absorption cross-section) for pure compound of NOMS were studied at (6.29) pH values of the medium. The optical transition probabilities were measured by integrating the absorption coefficient and molar decadic absorption coefficients in the wave number regions of 27173.9 - 37650.6 cm-1. Usually the UV-Vis spectrometer measures the concentration in terms of absorbance versus wavelength; this was recalculated into absorption coefficient or molar decadic absorption coefficient versus wave number using Origin 8 software and finally the average of the optical transition probabilities in a concentration range (1.65 - 2.71)10-2 were recorded. Similarly, the thermodynamic parameters such as enthalpy, Gibbs free energy and entropy of the self-association NOMS were studied at the temperature range (295 –312)K. These parameters have been determined using the model of Vant’s Hoff’s equation by linear curve fitting for the experimental data with that of the experimental values.
3. Results and Discussion
Self-Association of Neomycin Sulfate The experimental spectra of NOMS was studied in a concentration range of (3.1 - 1.65)10-2M using a bi-distilled water solvent at room Temperature [Fig. 2], and its quantitative analysis was carried out using the concentration dependent molar extinction coefficient of the drug molecule on the monomer form at absorption maximum of 304.8 nm. For the case of numerical analysis dimer model was derived by considering the following molecular equilibrium in solutions (Belay, 2012; Belay, 2010).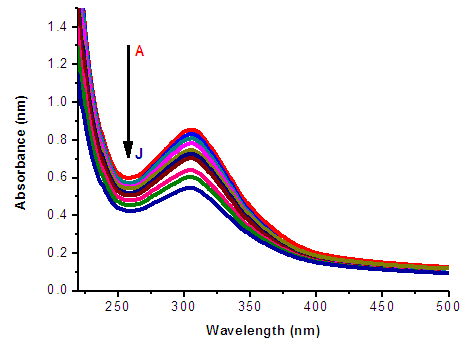 | Figure 2. Absorbance of Neomycin Sulfate A – J of concentration 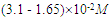 |
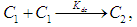 | (1) |
where 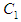 and
and 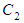 and
and 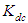 are monomers, dimers and the equilibrium dimerization constant of the drug respectively. The mass conservation law of the overall concentration of the dissolved molecules in solution is,
are monomers, dimers and the equilibrium dimerization constant of the drug respectively. The mass conservation law of the overall concentration of the dissolved molecules in solution is,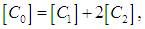 | (2) |
where 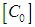 is the total concentration and
is the total concentration and 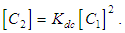 The contribution of the monomer and dimer to the molar extinction coefficient, of the solution is commonly considered to be additive, and
The contribution of the monomer and dimer to the molar extinction coefficient, of the solution is commonly considered to be additive, and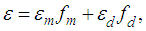 | (3) |
where 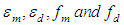 are molar monomer extinction coefficients, molar dimer extinction coefficients, equilibrium mole fraction of the molecules in the monomer concentration and equilibrium mole fraction of the molecules in the dimer concentration. And also,
are molar monomer extinction coefficients, molar dimer extinction coefficients, equilibrium mole fraction of the molecules in the monomer concentration and equilibrium mole fraction of the molecules in the dimer concentration. And also,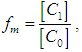
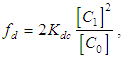 The dimer model for this can be obtained using Eq. 2 and Eq. 3,
The dimer model for this can be obtained using Eq. 2 and Eq. 3, 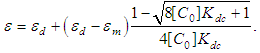 | (4) |
The unknown parameters 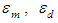 and
and 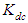 of Eq. 4 can be obtained by fitting this dimer model equation to the experimental data shown in Fig. 3 by Nonlinear curve fitting based on the Levenberg-Marquardt algorithm using origin 8 software. They are serving as search parameters being adjusted in order to achieve the minimum discrepancy between the experimental data and the theoretical value of Eq. 4. The values of molar extinction coefficients of Neomycin Sulfate at the maximum wavelength decrease as the concentration increases as shown in Fig. 3. The calculated association constants
of Eq. 4 can be obtained by fitting this dimer model equation to the experimental data shown in Fig. 3 by Nonlinear curve fitting based on the Levenberg-Marquardt algorithm using origin 8 software. They are serving as search parameters being adjusted in order to achieve the minimum discrepancy between the experimental data and the theoretical value of Eq. 4. The values of molar extinction coefficients of Neomycin Sulfate at the maximum wavelength decrease as the concentration increases as shown in Fig. 3. The calculated association constants 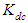 obtained at the wavelength of 304.8 nm is 1.06105 M-1. And also the calculated values of the monomer and dimer extinction coefficients are 1.239103 M-1 and 12.02M-1.cm-1 respectively. The obtained values are quite reasonable and comparable with the results obtained by (Xue et al., 2010) for intercalator-neomycin conjugates, with dimerization constant (2.4±0.1)105 M-1 at pH of 5.5.
obtained at the wavelength of 304.8 nm is 1.06105 M-1. And also the calculated values of the monomer and dimer extinction coefficients are 1.239103 M-1 and 12.02M-1.cm-1 respectively. The obtained values are quite reasonable and comparable with the results obtained by (Xue et al., 2010) for intercalator-neomycin conjugates, with dimerization constant (2.4±0.1)105 M-1 at pH of 5.5. 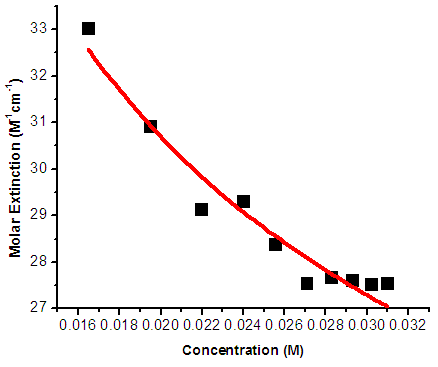 | Figure 3. Molar extinction Coefficient vs Concentration of NOMS Absorption Maxima of 304.8 nm |
Fig. 4, shows the mole fraction of monomer and dimer versus concentration of Neomycin sulfate drug molecules under the peak of 304.8 nm. The graphs show increase and decrease in the mole fraction of dimer and monomer as the concentrations of the drugs increases. The results indicated dimerization is favored at high concentration of the drugs.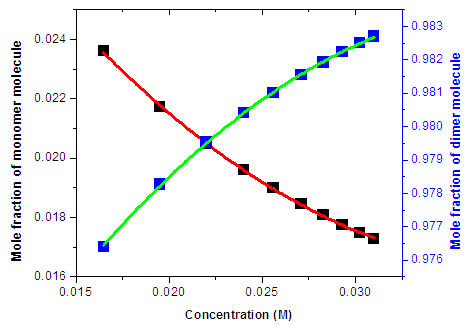 | Figure 4. The Mole Fraction of Monomer and Dimer versus Total Concentration of Neomycin Sulfate under the peak of 304.8 nm |
Optical transition properties of NOMSThe average optical transition probabilities of NOMS in a concentration range (1.65 - 2.71)10-2 were obtained from the absorption spectra to characterize the strength of the electron transition and to interpret the absorption spectra. The molar decadic absorption coefficient which represents the ability of a molecule to absorb light in a given solvent at a given wavelength was calculated using Beer-Lambert’s law (Liptay, 1969; Belay, 2013) and integrated absorption coefficient is the sum of absorption coefficient for all frequencies. It is independent of line function which may be varying due to pressure, temperature, interaction of solute and solvent (Milonni and Eberly, 1988; Michale, 1999). The integrated absorption coefficient 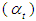 in the certain frequency (v) ranges can be expressed by,
in the certain frequency (v) ranges can be expressed by,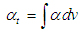 | (5) |
From Eq. (5) the integrated absorption cross-section 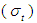 is given by (Milonni and Eberly, 1988);
is given by (Milonni and Eberly, 1988);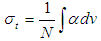 | (6) |
Where, 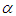 absorption coefficient and N is number density of the molecules.Oscillator strength which represents the average number of electrons per atom that can be excited by the incident radiation is important parameter for providing the relative strength of electron transition and can be related as below with the molar decadic absorption coefficient
absorption coefficient and N is number density of the molecules.Oscillator strength which represents the average number of electrons per atom that can be excited by the incident radiation is important parameter for providing the relative strength of electron transition and can be related as below with the molar decadic absorption coefficient 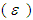 as a function of frequency (Georgakopoulous et al., 2004);
as a function of frequency (Georgakopoulous et al., 2004);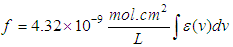 | (7) |
The transition dipole moment 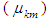 is a vector that depends on both ground state and excited state and couple the transition to the electric field of light and it is related with molar decadic absorption coefficient as (Liptay, 1969; Michale, 1999).
is a vector that depends on both ground state and excited state and couple the transition to the electric field of light and it is related with molar decadic absorption coefficient as (Liptay, 1969; Michale, 1999).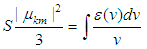 | (8) |
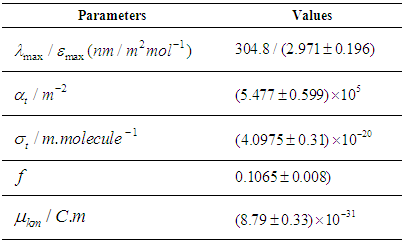
Where 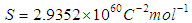 . Using the above equations the average value of optical transition properties of NOMS in a concentration range (1.65 - 2.71)10-2 calculated in bi-distilled water are presented in Table 1. The peak in the visible region of NOMS is due to
. Using the above equations the average value of optical transition properties of NOMS in a concentration range (1.65 - 2.71)10-2 calculated in bi-distilled water are presented in Table 1. The peak in the visible region of NOMS is due to  electronic transitions of chromophore groups (Bakhkshiev, 1961; Firth et al., 1983). Thermodynamic Properties of the Self-Association of Neomycin Sulfate Heating the aqueous solution of Neomycin Sulfate shows that the absorption spectra of the molecules are strongly dependent on the temperature in the range of (295 –312)K. The equilibrium constants of the drug molecules at the above mentioned temperature were calculated at peak of wavelengths of the self-association using Eq.(3) respectively. Fig. (5), shows the variation of versus
electronic transitions of chromophore groups (Bakhkshiev, 1961; Firth et al., 1983). Thermodynamic Properties of the Self-Association of Neomycin Sulfate Heating the aqueous solution of Neomycin Sulfate shows that the absorption spectra of the molecules are strongly dependent on the temperature in the range of (295 –312)K. The equilibrium constants of the drug molecules at the above mentioned temperature were calculated at peak of wavelengths of the self-association using Eq.(3) respectively. Fig. (5), shows the variation of versus 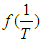 . The magnitude of the enthalpy was estimated from the slope of the approximating line according to Vant’s Hoff’s equation (Belay, 2010):
. The magnitude of the enthalpy was estimated from the slope of the approximating line according to Vant’s Hoff’s equation (Belay, 2010):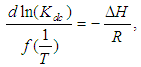 | (9) |
where ∆H is the molar enthalpy change, 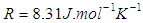 is the universal gas constant and T the temperature in Kelvin. The entropy was derived from Gibbs free energy and enthalpy. The relation between Gibbs free energy and entropy can be expressed as
is the universal gas constant and T the temperature in Kelvin. The entropy was derived from Gibbs free energy and enthalpy. The relation between Gibbs free energy and entropy can be expressed as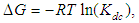 | (10) |
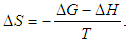 | (11) |
Finally, the Vant’s Hoff’s equation can be given by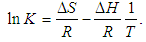 | (12) |
Plots of 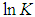 versus
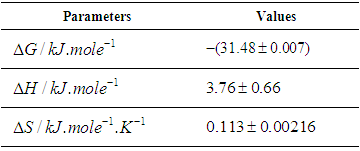
versus 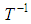 gives a straight line, whose slope and intercept can be used to determine ∆S and ∆H and Gibb’s free energy can be determined at a specific temperature using Eq. (11). In order to characterize the force between NOMS molecules, thermodynamic parameters on the given temperatures were analyzed using Vant’s Hoff’s equation. The thermodynamic parameters, Gibb’s free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) are important for confirming the binding mode. The calculated value for the Gibbs free energy, enthalpy, and entropy of the drug molecules at a temperature (295 –312)K are given in table (2). Since ΔH >0 and ΔS >0, hydrophobic interactions are dominant for the interaction of NOMS molecules (Liu et al., 2014). Moreover, the negative value for the Gibb’s free energy indicates that the absorption process of the drug is continuous. Also, the positive value of entropy confirms the increasing randomness of the solution interface during the absorption process of the drug molecules (Liu et al., 2014).The obtained thermodynamic parameters for NOMS are quite reasonable and comparable with the result binding of neomycin with BSA obtained by (Keswani et al., 2010), the results are,
gives a straight line, whose slope and intercept can be used to determine ∆S and ∆H and Gibb’s free energy can be determined at a specific temperature using Eq. (11). In order to characterize the force between NOMS molecules, thermodynamic parameters on the given temperatures were analyzed using Vant’s Hoff’s equation. The thermodynamic parameters, Gibb’s free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) are important for confirming the binding mode. The calculated value for the Gibbs free energy, enthalpy, and entropy of the drug molecules at a temperature (295 –312)K are given in table (2). Since ΔH >0 and ΔS >0, hydrophobic interactions are dominant for the interaction of NOMS molecules (Liu et al., 2014). Moreover, the negative value for the Gibb’s free energy indicates that the absorption process of the drug is continuous. Also, the positive value of entropy confirms the increasing randomness of the solution interface during the absorption process of the drug molecules (Liu et al., 2014).The obtained thermodynamic parameters for NOMS are quite reasonable and comparable with the result binding of neomycin with BSA obtained by (Keswani et al., 2010), the results are, 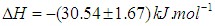 and
and 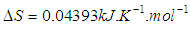 .
. 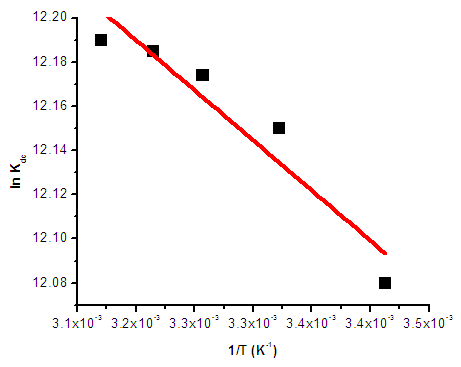 | Figure 5.  of NOMS at Concentration of NOMS at Concentration 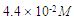 |
Table 1. Calculated result of the optical transition Properties of NOMS in a concentration range (1.65 - 2.71) 10-2mole.L-1
 |
| |
|
Table 2. Calculated result of the thermodynamic Parameters of NOMS in a temperature range (295 –312)K
 |
| |
|
4. Conclusions
The findings of this research indicate that the molecule of Neomycin Sulfate interacts with itself due to hydrophobic interaction. The calculated parameters for the self-association and thermodynamic properties of the compound are interpreting the study of kinetic chemical reaction system of the compound, and also the thermodynamic parameters which are derived from the temperature dependency of the dimerization constant, have given insight in to the forces that maintain the dimer structures in the solution. The other, optical transition probabilities are useful for understanding the nature and strength of its molecular interaction in liquid solutions, in order to characterize the electron transition probabilities and interpret the absorption spectra of the compound, for direct experimentally application in the emission, absorption and dispersion, and in providing stringent test of atomic and molecular structure calculation in theoretical works. Thus, knowledge of the mechanism of self-association of NOMS is useful in order to design the advanced and controllable carriers of drugs and food components. Therefore, the investigated results have wider applications in pharmaceutical and food companies in terms of economic and scientific utility.
ACKNOWLEDGMENTS
The authors wish to acknowledge the generous grant by International Science program (ISP) of Uppsala University, Uppsala, Sweden. We are grateful to Dr. Mulugeta for providing facilities at polymer Laboratory of Physics Department, CNS, AAU.
References
| [1] | Bayliss, N.S. 1950. The effect of electrostatic polarization of solvent on electronic absorption spectra in solution, J.Chem.Phys., 18: 292 |
| [2] | Bakhkshiev, N.G. 1961. Universal intermolecular interactions and their effects on the position and electronic spectra of molecules in two components solutions, Optic. Spectrosc. 10:379-384. |
| [3] | Belay A (2012). Spectrophotometric Method for the Determination of Caffeic Acid Complexation and Thermodynamic Properties. Int. J. Biophys. 2, 12-17. |
| [4] | Belay A (2010). Determination of self - associated 5- caffeoylquinic acid and its complexation with sodium hydroxide using UV-Vis spectroscopy. Int. J. Phys. Sci. 5(5): 459-464. |
| [5] | Belay A (2013). Determination the Optical Transition Probabilities and Number Densities of Ethidium Bromide (EB) in Hetero-Association by Integrated Absorption Coefficient Techniques. J. Biol. Chem. Research. Vol. 30, No. 2: 451-465 |
| [6] | Darwhekar G, Jain DK, Choudhary A (2012). Elastic liposomes for delivery of neomycin sulphate in deep skin infection. Asian J. Pharm. Sci., 7 (4): 230-240. |
| [7] | Firth, W.J., Watkins, C.L., Graves, D.E. and Yielding, L.W. 1983. Synthesis and characterization of ethidium analogs, emphasis on amino and azido substituent, J. Heterocycl. Chem., 20:759-765. |
| [8] | Georgakopoulous S, van Grondelle R, van der Zwan G (2004). Circular dichroism of Carotenoides in bacterial light-harvesting complexes: experimental and modeling. Biophy. J. 87: 3010-3022. |
| [9] | Guo Y, Liu B, Li Z, Zhang L, Lv Y (2014). Study of the combination reaction between drugs and bovine serum albumin with methyl green as a fluorescence probe. J. Chem. Pharm. Res., 6(5), 968-974. |
| [10] | Keswani N, Choudhary S, Kishore N (2010). Interaction of weakly bound antibiotics neomycin and lincomycin with bovine and human serum albumin: biophysical approach. J. Biochem. 148(1):71–84. |
| [11] | Liptay W (1969). Electrochromism and solvatochromism. Angew. Chem.Int. Ed. Engl. 8: 177-187. |
| [12] | Mac Rae, G.E. 1957. Theory of solvent effects on molecular electronic spectra, J.Phy. Chem., 61: 562. |
| [13] | Michale JL (1999). Molecular spectroscopy. USA: Prentice-Hall Inc. |
| [14] | Milonni, P.W. and Eberly, J.H. 1988. Lasers. Wiely, New York |
| [15] | Niazi A, Yazdanipour A, Ghasemi J. Kubista M (2006). Spectrophotometric and thermodynamic study on the dimerization equilibrium of ionic dyes in water by chemometrics method, Spectrochimica Acta Part A 65: 73–78. |
| [16] | Patel AH, Dave RM (2015). Formulation and evaluation of sustained release in situ ophthalmic gel of neomycin sulphate. Bull. Pharm. Res., 5(1): 1-5. |
| [17] | Sunil Kumar, P. Yousaf Khan, N. K. Verma, S. K. Chakarvarti (2008). Optical Parameters of Znse Chalcogenide Nanostructures. Chalcogenide Letters Vol. 5, No. 7, 143 – 152. |
| [18] | Waksman S A, Katz E, Lechevalier H A (1950). Antimicrobial properties of Neomycin. J. Lab. Clin. Med. 36: 93–99. |
| [19] | Xue L, Xi H, Kumar S, Gray D, Davis E, Hamilton P, Skirba M, Arya DP (2010). Probing the recognition surface of a DNA triplex: Binding studies with intercalator-neomycin conjugates. Biochemistry., 49(26): 5540–5552. |
| [20] | Zejc A, Gorczyca M (2002). Chemistry of Drugs. Polish, PZWL, Warszawa. |




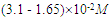
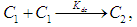
 and
and  and
and 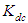 are monomers, dimers and the equilibrium dimerization constant of the drug respectively. The mass conservation law of the overall concentration of the dissolved molecules in solution is,
are monomers, dimers and the equilibrium dimerization constant of the drug respectively. The mass conservation law of the overall concentration of the dissolved molecules in solution is,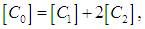
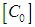 is the total concentration and
is the total concentration and 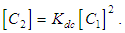 The contribution of the monomer and dimer to the molar extinction coefficient, of the solution is commonly considered to be additive, and
The contribution of the monomer and dimer to the molar extinction coefficient, of the solution is commonly considered to be additive, and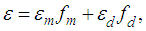
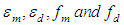 are molar monomer extinction coefficients, molar dimer extinction coefficients, equilibrium mole fraction of the molecules in the monomer concentration and equilibrium mole fraction of the molecules in the dimer concentration. And also,
are molar monomer extinction coefficients, molar dimer extinction coefficients, equilibrium mole fraction of the molecules in the monomer concentration and equilibrium mole fraction of the molecules in the dimer concentration. And also,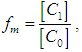
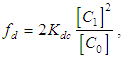 The dimer model for this can be obtained using Eq. 2 and Eq. 3,
The dimer model for this can be obtained using Eq. 2 and Eq. 3, 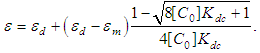
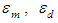 and
and 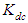 of Eq. 4 can be obtained by fitting this dimer model equation to the experimental data shown in Fig. 3 by Nonlinear curve fitting based on the Levenberg-Marquardt algorithm using origin 8 software. They are serving as search parameters being adjusted in order to achieve the minimum discrepancy between the experimental data and the theoretical value of Eq. 4. The values of molar extinction coefficients of Neomycin Sulfate at the maximum wavelength decrease as the concentration increases as shown in Fig. 3. The calculated association constants
of Eq. 4 can be obtained by fitting this dimer model equation to the experimental data shown in Fig. 3 by Nonlinear curve fitting based on the Levenberg-Marquardt algorithm using origin 8 software. They are serving as search parameters being adjusted in order to achieve the minimum discrepancy between the experimental data and the theoretical value of Eq. 4. The values of molar extinction coefficients of Neomycin Sulfate at the maximum wavelength decrease as the concentration increases as shown in Fig. 3. The calculated association constants 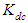 obtained at the wavelength of 304.8 nm is 1.06105 M-1. And also the calculated values of the monomer and dimer extinction coefficients are 1.239103 M-1 and 12.02M-1.cm-1 respectively. The obtained values are quite reasonable and comparable with the results obtained by (Xue et al., 2010) for intercalator-neomycin conjugates, with dimerization constant (2.4±0.1)105 M-1 at pH of 5.5.
obtained at the wavelength of 304.8 nm is 1.06105 M-1. And also the calculated values of the monomer and dimer extinction coefficients are 1.239103 M-1 and 12.02M-1.cm-1 respectively. The obtained values are quite reasonable and comparable with the results obtained by (Xue et al., 2010) for intercalator-neomycin conjugates, with dimerization constant (2.4±0.1)105 M-1 at pH of 5.5. 

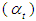 in the certain frequency (v) ranges can be expressed by,
in the certain frequency (v) ranges can be expressed by,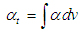
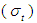 is given by (Milonni and Eberly, 1988);
is given by (Milonni and Eberly, 1988);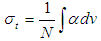
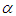 absorption coefficient and N is number density of the molecules.Oscillator strength which represents the average number of electrons per atom that can be excited by the incident radiation is important parameter for providing the relative strength of electron transition and can be related as below with the molar decadic absorption coefficient
absorption coefficient and N is number density of the molecules.Oscillator strength which represents the average number of electrons per atom that can be excited by the incident radiation is important parameter for providing the relative strength of electron transition and can be related as below with the molar decadic absorption coefficient 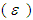 as a function of frequency (Georgakopoulous et al., 2004);
as a function of frequency (Georgakopoulous et al., 2004);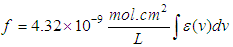
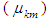 is a vector that depends on both ground state and excited state and couple the transition to the electric field of light and it is related with molar decadic absorption coefficient as (Liptay, 1969; Michale, 1999).
is a vector that depends on both ground state and excited state and couple the transition to the electric field of light and it is related with molar decadic absorption coefficient as (Liptay, 1969; Michale, 1999).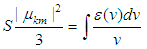
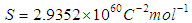 . Using the above equations the average value of optical transition properties of NOMS in a concentration range (1.65 - 2.71)10-2 calculated in bi-distilled water are presented in Table 1. The peak in the visible region of NOMS is due to
. Using the above equations the average value of optical transition properties of NOMS in a concentration range (1.65 - 2.71)10-2 calculated in bi-distilled water are presented in Table 1. The peak in the visible region of NOMS is due to 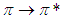 electronic transitions of chromophore groups (Bakhkshiev, 1961; Firth et al., 1983). Thermodynamic Properties of the Self-Association of Neomycin Sulfate Heating the aqueous solution of Neomycin Sulfate shows that the absorption spectra of the molecules are strongly dependent on the temperature in the range of (295 –312)K. The equilibrium constants of the drug molecules at the above mentioned temperature were calculated at peak of wavelengths of the self-association using Eq.(3) respectively. Fig. (5), shows the variation of versus
electronic transitions of chromophore groups (Bakhkshiev, 1961; Firth et al., 1983). Thermodynamic Properties of the Self-Association of Neomycin Sulfate Heating the aqueous solution of Neomycin Sulfate shows that the absorption spectra of the molecules are strongly dependent on the temperature in the range of (295 –312)K. The equilibrium constants of the drug molecules at the above mentioned temperature were calculated at peak of wavelengths of the self-association using Eq.(3) respectively. Fig. (5), shows the variation of versus 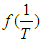 . The magnitude of the enthalpy was estimated from the slope of the approximating line according to Vant’s Hoff’s equation (Belay, 2010):
. The magnitude of the enthalpy was estimated from the slope of the approximating line according to Vant’s Hoff’s equation (Belay, 2010):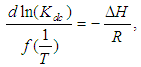
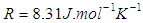 is the universal gas constant and T the temperature in Kelvin. The entropy was derived from Gibbs free energy and enthalpy. The relation between Gibbs free energy and entropy can be expressed as
is the universal gas constant and T the temperature in Kelvin. The entropy was derived from Gibbs free energy and enthalpy. The relation between Gibbs free energy and entropy can be expressed as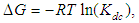
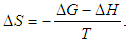
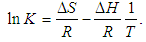
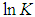 versus
versus 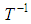 gives a straight line, whose slope and intercept can be used to determine ∆S and ∆H and Gibb’s free energy can be determined at a specific temperature using Eq. (11). In order to characterize the force between NOMS molecules, thermodynamic parameters on the given temperatures were analyzed using Vant’s Hoff’s equation. The thermodynamic parameters, Gibb’s free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) are important for confirming the binding mode. The calculated value for the Gibbs free energy, enthalpy, and entropy of the drug molecules at a temperature (295 –312)K are given in table (2). Since ΔH >0 and ΔS >0, hydrophobic interactions are dominant for the interaction of NOMS molecules (Liu et al., 2014). Moreover, the negative value for the Gibb’s free energy indicates that the absorption process of the drug is continuous. Also, the positive value of entropy confirms the increasing randomness of the solution interface during the absorption process of the drug molecules (Liu et al., 2014).The obtained thermodynamic parameters for NOMS are quite reasonable and comparable with the result binding of neomycin with BSA obtained by (Keswani et al., 2010), the results are,
gives a straight line, whose slope and intercept can be used to determine ∆S and ∆H and Gibb’s free energy can be determined at a specific temperature using Eq. (11). In order to characterize the force between NOMS molecules, thermodynamic parameters on the given temperatures were analyzed using Vant’s Hoff’s equation. The thermodynamic parameters, Gibb’s free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) are important for confirming the binding mode. The calculated value for the Gibbs free energy, enthalpy, and entropy of the drug molecules at a temperature (295 –312)K are given in table (2). Since ΔH >0 and ΔS >0, hydrophobic interactions are dominant for the interaction of NOMS molecules (Liu et al., 2014). Moreover, the negative value for the Gibb’s free energy indicates that the absorption process of the drug is continuous. Also, the positive value of entropy confirms the increasing randomness of the solution interface during the absorption process of the drug molecules (Liu et al., 2014).The obtained thermodynamic parameters for NOMS are quite reasonable and comparable with the result binding of neomycin with BSA obtained by (Keswani et al., 2010), the results are, 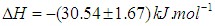 and
and 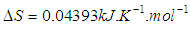 .
. 
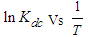 of NOMS at Concentration
of NOMS at Concentration 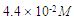
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
