-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biophysics
p-ISSN: 2168-4979 e-ISSN: 2168-4987
2015; 5(1): 18-23
doi:10.5923/j.biophysics.20150501.03
A Description of the Effect of Polarized Light as an Adjuvant Therapy on Wound Healing Process in Pediatrics
Samir M. Abdel-Mageed1, Ali Osman Selim2, Mohamed A. Abdel Ghafar3, Rania Reffat Ali4
1Physics Department, Faculty of Science, Alexandria University
2Physical Therapy for Surgery Department, Faculty of Physical Therapy, Cairo University
3Physical Therapy for Pediatrics Department, Faculty of Physical Therapy, October 6 University
4Basic science Department, Faculty of Physical Therapy, Cairo University
Correspondence to: Samir M. Abdel-Mageed, Physics Department, Faculty of Science, Alexandria University.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Backgroundandpurpose: The ability of light to penetrate a tissue and deposit energy via the optical absorption properties of the tissue is the key to therapeutic applications. Birefringence which is an anisotropic property of dermal layer of skin, the blood flow in the tissue capillaries and the multi-scattering from the tissue static components affect the polarization of light. The goal of this study was to describe the healing process of deep partial thickness wound in pediatrics submitted (or not) to the excitation property or the polarization property of light.Subjects: Thirty children who suffered from deep partial thickness burn (20-30%) participated in this study (13 boys and 17 girls). Their ages ranged from 10 to18 years. They were classified randomly into two groups (study and control group) of equal numbers.Procedures: Group A (study) was treated using polarized light as an adjuvant therapy to the regular wound care (debridement, Local antimicrobial drug and beta dine) 5 sessions per week for 3 weeks. Group B (control) was treated using the regular wound care (debridement, Local antimicrobial drug and beta dine). Wound surface areas were measured before and after the treatment period by using wound tracing method. Results: The study showed a significant reduction in burn wound surface areas in both groups (P < 0.05) while there was no significant difference between both groups.Conclusion:it was concluded that polarized light has a limited effect as an adjuvant therapy on healing process of deep partial thickness burn wounds in children.
Keywords: Polarized light, Deep partial thickness burn, Wound healing, Paediatrics
Cite this paper: Samir M. Abdel-Mageed, Ali Osman Selim, Mohamed A. Abdel Ghafar, Rania Reffat Ali, A Description of the Effect of Polarized Light as an Adjuvant Therapy on Wound Healing Process in Pediatrics, International Journal of Biophysics , Vol. 5 No. 1, 2015, pp. 18-23. doi: 10.5923/j.biophysics.20150501.03.
1. Introduction
- Burn is a coagulative necrosis of the skin caused by contact with fire, chemicals such as acids, hot liquids, hot air and hot gases, heated metals or electricity. Burn injuries remain one of the major health problems that can happen unexpectedly and have the potential to cause disability, lifelong disfigurement, prolonged hospitalization and/or death. [1, 2]Unintentional and intentional burn injuries vary across age groups, gender, income and global region. The worldwide incidence of burn injuries was 1.3 per 100,000 populations in low and moderate income countries. Children under 5 and the elderly have the highest burn mortality worldwide. [3]The repair of burn wound has long been a problem and this has come more into focus in recent times. Wound closure is undertaken as the most important advent preventing life threatening, infections, decreasing morbidity and mortality [7]. Wound healing is an intricate process in which the skin or any organ tissue repairs itself after injury [4]. Wound healing is a complex and dynamic process characterized by interaction of various cell types as lymphocytes, monocytes, epithelial cells and fibroblasts. Three main overlapping phases have been identified in tissue response to injury include inflammation, formation of granulation tissue and proliferation [5, 6]. Wound healing process is based on the vascular and cellular activity. Vasomotion is the periodic constriction and dilatation of small blood vessels. It is attributed to local metabolic needs, vascular myogenic responses and neurogenic controls. Besides the fibroblast and macrophage activity, human wound associated lymphocyte populations are modulated during a healing process. [4]Polarized and non-polarized light from different sources in the spectral ranges of visible and near infrared (NIR) are widely used in clinical treatments and in medical research especially in the therapeutic field, such as Low Level Laser Therapy (LLLT), light phototherapy (lasers), quasi-monochromatic radiation (LEDs) and broadband light devices. [8] The most of laser types are polarized, where the electric field oscillates in a certain direction perpendicular to the propagation direction [9].Light is a form of energy and has various colors with different wavelength; it has been used as a healing tool since ancient time. There is now better understanding of which component of natural light are useful in the stimulation of healing such as Biptron light therapy (BLT) which is a device emits a polarized light containing a range of wavelengths that correspond to visible light plus infrared radiation which have been reported to stimulate the biological reactions [10]. It was reported that polarized light causes enhancement of the cell membrane activities, acceleration of production of adenosine tri-phosphate (ATP) in mitochondria, stimulation of regeneration processes and acceleration of fibroblast proliferation and deposition of collagen [11, 12].It was considered that polarized light rearrange the polar heads of a lipid bilayer in the cell membrane where enzyme reactions take place lead to structural changes occur in cell membranes in consequence the surface features and lipid protein connection can be modified [11]. Schindl et al. [13] stated that Biptron light therapy may significantly stimulate the faster epithelialization of the damaged skin, reducing the risk for the formation of the functionally and unacceptable scars. However, the polarization point has never been experimentally proven and studies used polarized light in the treatment of wounds revealed conflicting data and studies in vivo are still scarce, with some studies reporting accelerated wound closure and increased tensile strength of scars while others have found no such improvement [14]. So, the purpose of this study was to evaluate the effect of polarized light in accelerating the healing rate in burn injuries in children.
2. Materials and Methods
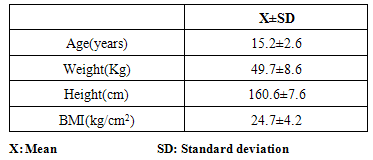
- Subjects: Thirty children (13 boys and17 girls) participated in this study; their ages ranged from 10 to 18 years with a mean value of 15.2±2.6 years. The mean values for their body weight was 49.7±8.6 kg, and for their height was 160.6±7.6 cm, while it was 24.7±4.2 kg/cm2 for their body mass index (Table 1). A consent form was signed after the procedure and possible side effects had been explained under supervision of their guardians.
|
3. Results
- The statistical analysis of the mean values of wound surface area (WSA) in control group before treatment was (105.7±20.96) and after treatment was (17.2±11.49) revealed a significant statistical reduction of mean values of WSA(P<0.05). Also the percentage of reduction was calculated to be 88.3% after treatment, when compared to the pretreatment value as shown in figure 1.
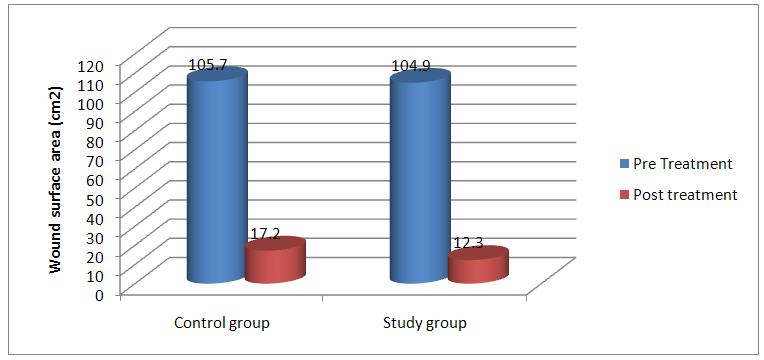 | Figure 1. The statistical analysis of mean values of WSA pre treatment after post treatment in control and study groups |
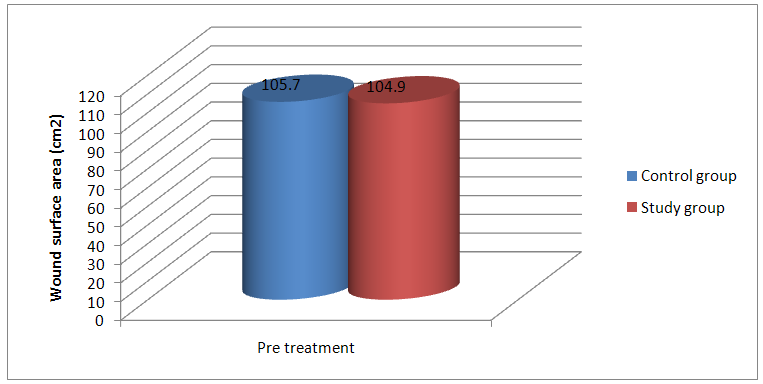 | Figure 2. The Mean values of WSA (cm2) post treatment for both control and study groups |
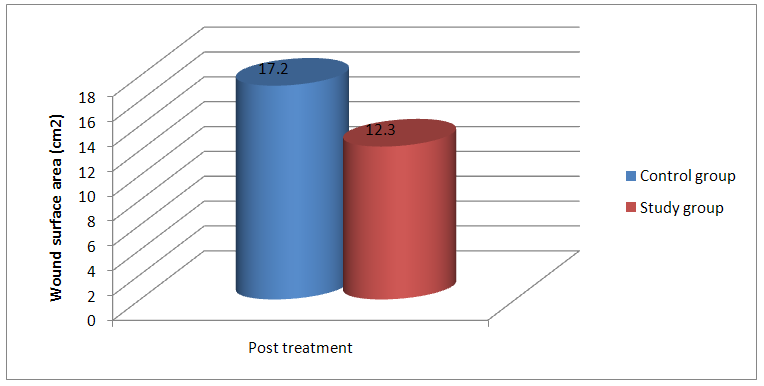 | Figure 3. The Mean values of WSA (cm2) post treatment for both control and study groups |
4. Discussion
- The analysis of the results indicated that both groups showed significant statistical difference between pre and post experiment measurements, but there was no statistically significant difference between the study and the control groups.Results of the current study came in agreement with those of Schlager et al. [16] who reported that irradiation of burns with a 250-mW/670-nm laser light produced no beneficial effects on wound-healing processes. Also it is supported by Cambier et al. [17] who found no improvement in the wound-healing process after irradiation with low-power lasers or polarized light.These results can be explained as the therapeutic effect of polarized light may be due to either the polarization or the excitation property of the light. Once the polarized lights penetrate the tissues it starts to lose their polarization direction. The polarization loss in the tissue may be mainly due to many reasons; the blood flow in the tissue capillaries, the birefringent properties of the medium and the multi-scattering from the tissue static components [18].First of all, according to the work of Fixler et al. [19] the polarization loss is directly proportional to the volumetric flow rate. Higher flow rate produces lower polarization values especially when the polarization direction is perpendicular to the direction of flow. The dermis layer of the skin - first layer encountered by the polarized light during second degree burn wound contains blood vessels and capillaries where the blood is flowing in many directions carrying its components. Accordingly, the polarized light beam necessarily hits the fluid flow through the tissue perpendicular to its polarization direction and this cause a polarization loss.Secondly, the randomization of linear polarization during transmission through a tissue is more rapid in birefringent tissues than in non-birefringent tissues [20]. Birefringence is a polarization specific electromagnetic property of materials. Collagen fiber is the main components of the dermis layer (70% in dry weight) has a heterogeneous distribution in the skin, and commonly refer to as birefringent tissue [21]. There are two types of birefringence have been reported for collagen fibers, particularly intrinsic [22] and Form birefringence which is caused by asymmetrical alignment of chemical bonds or ions within the rod shaped triple chain collagen molecules and the next arises from the nonlinear optical property of the medium [23]. Therefore, Collagen’s rod like triple helix conformation results in both linear and circular anisotropic properties [24]. The most rapid depolarization occurred in rich collagen tissues of the skin [20].Finally, on the cellular level, the mismatches of refractive indices between cellular components result in scattering [25]. The scattering properties of the cellular level elements also vary with their sizes. Therefore, the reduced scattering coefficient of the dermis as a function of the light wavelength can be described well as a combination of Mie and Rayleigh scattering. The former occur when the size of the element is comparable to the wavelength of the incident light such as scattering by mitochondria, nuclei and collagen fibers can be explained by Mie scattering. Contrary, when the size of the element is much smaller than the wavelength, its scattering property can be explained by Rayleigh scattering such as scattering include membranes and the banded ultra-structures of collagen fibrils fibers [26]. Therefore; light with long wavelength has more penetration power in skin. Rayleigh scattering dominates in the spectral range below 650 nm while Mie scattering plays a major role above 650nm. As the main scatterers in the dermis are the collagen fibers which are densely packed [27]. As a result, the orientation of polarization becomes randomized by multiple scattering events from these collagen fibers and the polarization value reduced [28]. Based on the three facts discussed above ; the dermis static components such as collagen fibers exhibit optical birefringent properties which acts as a strong multiple scatterers for linear polarized light, also the blood flow in the tissue capillaries and the blood dynamic components act as a scatterers. Consequently, linear polarization of radiation randomized through the dermis layer and polarization lost. So there are no additional benefits in using polarized light as an excitation source without maintaining the excitation direction.The slight improvement in the group treated by polarized light compared to the control group comes in agreement with Ribeiro [29] who reported that the relative direction of the laser polarization plays an important role in the wound healing process when highly coherent He-Ne laser is used. Also these results confirmed by the clinical results of Monstrey et al. [30] that polarized light therapy was effective in the treatment of burn wounds.These results may be attributed to the excitation property of the light despite of its polarization property. The light interaction with the biological tissue is not an intrinsic property of the light. Where the first law of photochemistry states that light must be absorbed before photochemistry can occur.It was stated that mitochondria thought to be a likely site for the initial effects of light; absorption of photons by chromosphere molecules such as cytochrome c that proposed as the primary photoacceptor for the red-NIR range in mammalian cells leads to electronically excited states and consequently acceleration of electron transfer reactions in the mitochondria membrane respiratory chain [31]. A further electron transport automatically leads to increasing of ATP production rate [32], alteration of reactive oxygen species and induction of transcription factors which increasing the activity of the Na+ /H+ and Ca2+/Na+ antiporters and of all the ATP driven carriers for ions, such as Na+ /K+ ATPase and Ca2+ pumps. ATP is the substrate for adenyl cyclase, and therefore the ATP level controls the level of cAMP. Both Ca2+ and cyclic adenosine mono-phosphate cAMP are very important second messengers. Ca2+ adjusts most of the biological process in the human body [31].The positive effect of light on wound healing can be explained by many biological mechanisms including modulation in levels of cytokines and growth factors which are responsible for the different stages of wound healing. It was reported that cytokines responsible for the initial inflammatory phase, fibroblast proliferation and migration in wound healing [33]. Also it was confirmed that light can increase growth factors responsible for the neovascularization and inducing collagen synthesis from fibroblasts which necessary for wound healing [34]. Light can incite fibroblasts transformation to myofibloblasts which has the phenotype of contractile cells that accelerate wound contraction [35].
5. Conclusions
- The effects of polarized light as an adjuvant therapy for deep partial thickness second degree burn were not satisfactory and statistically non-significant. But it showed a very slight improvement in the wound healing process.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML