-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Archaeology
p-ISSN: 2332-838X e-ISSN: 2332-841X
2021; 9(1): 56-67
doi:10.5923/j.archaeology.20210901.11

Study and Conservation of a 19th Century Printed Silk Scarf from the Collection of the National Historical Museum of Greece
Nikoleta Tasiouli 1, Stamatis Boyatzis 2, Anna Karatzani 2, Andreas G. Karydas 3
1Conservation Department, National Historical Museum, Athens, Greece
2Dept. of Conservation of Antiquities and Works of Art, University of West Attica, Athens, Greece
3Institute of Nuclear and Particle Physics, National Center for Scientific Research “DEMOKRITOS”, Athens, Greece
Correspondence to: Nikoleta Tasiouli , Conservation Department, National Historical Museum, Athens, Greece.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aiming at an optimal gel-based methodology for the selective removal of stains on a 19th c. ink-printed silk scarf depicting the temple of Holy Trinity Church of Vienna, its history and preservation condition was identified with the help of optical microscopy, UVFC imaging, gloss and pH measurements, colorimetry, X-ray Fluorescence (XRF) and Fourier Transform Infrared (FTIR) spectroscopy. Emphasis was given on non-invasiveness and selectivity, for zeroing, or minimizing the effects from the cleaning methodology on the base material (silk) and the print (oil binder-based carbon black). Based on the analytical results, printed silk mock-ups were developed with polysaccharide stains, foxing spots and acrylic glue residues. These were artificially aged to simulate the pathology and condition of the printed scarf. The results of the gel-systems cleaning applications on the mock-ups led to the selection of the appropriate gel systems for the selective cleaning of the printed scarf: Αgar for polysaccharide deposits, Gellan gum-chelating factors (EDTA, DTPA) gel systems for foxing-metallic ions stains and Agar-enzyme lipase gel system for oxidized acrylic glue residues, were used. DEC-based PMMA and Nevek organogels, also used for the various tape backing detachment and adhesive removal from purpose made mock-ups. As enzyme, chelating factors, pH regulator (Trizma) and viscosity gel regulator (calcium acetate) were included in the gel formulations, their residues, after cleaning applications, were studied with infrared spectroscopy (ATR-FTIR) and ultraviolet induced visible fluorescence photography (UVFC).
Keywords: Silk, Gel systems, Selective stain removal, Chelators, Acrylic adhesive, Accelerated aging, FTIR/ATR, XRF, SEM/EDS, Optical microscopy, Non-destructive imaging (UVFC/VIS)
Cite this paper: Nikoleta Tasiouli , Stamatis Boyatzis , Anna Karatzani , Andreas G. Karydas , Study and Conservation of a 19th Century Printed Silk Scarf from the Collection of the National Historical Museum of Greece, Archaeology, Vol. 9 No. 1, 2021, pp. 56-67. doi: 10.5923/j.archaeology.20210901.11.
Article Outline
1. Introduction
- The advantages of using gel systems in conservation of works of art have been widely studied on easel paintings, paper art works, sculpture, wall paintings etc [1-6].However, very few articles were traced concerning gel applications on silk conservation treatments [7]. In this study, based on current knowledge concerning the use of gel systems on various works of art cleaning, a gel-based optimal methodology was developed for the selective removal of different kind of stains, deposits and oxidized acrylic glue residues from a printed silk scarf.Silk is a fragile material and is difficult to be restored once it is damaged. Structural changes due to ageing lead to a decrease in stability and in its tensile strength and a change in colour. Silk deterioration critical factors are: the effect of acids (pH<3,4), alkaline environment (pH>7), high ratios of relative humidity, UV radiation, effect of air pollutants such as Sulphur, as well as use and improper storage conditions [8]. The acidic conditions enable the acid hydrolysis of the peptide bonds. The amorphous region of the fibroin protein molecule is the first to be affected and tyrosine levels decrease. Due to alkaline conditions the chains of the amorphous regions are pushed apart and finally cleavage of salt and hydrogen bonds occur. Silk may bond considerable amounts of SO2 from the atmosphere. This is volatile unless oxidation occurs and due to moisture, H2SO4 may be formed. The above factors lead to the reduction of the pH, the mechanical strength and fragility of silk and in some cases in total loss of material [8]. Furthermore, silk yellowing is caused by the photooxidation of tyrosine and tryptophan residues, which results in the formation of yellow chromophores [9]. Additionally, silk weighted with inorganic salts of iron, tin and lead undergoes photodeterioration at a high rate, because the metals catalyse the photo-oxidative process [10,11,12,13].Silk is a hydrophilic material. In contact with water, silk fibres swell. In contrary, dehydrated silk is rigid, brittle and has decreased softness [8]. So, it is crucial to optimize the cleaning process in order to remove all contamination and degradation products to minimize invasive treatment that can cause irreversible damage.Silk treatment with gels, minimizes handling and selective cleaning can be achieved. The phenomenon of capillary diffusion is prevented when 3 to 5% w/v gel systems are applied on water soluble inks, watercolours or pigments. Gel can be easily applied to small areas of the fabric were stain and oxidation removal are achieved through the osmosis mechanism. Once applied, the gel allows the cleaning solution to diffuse out and bind to particles of dirt and soil in the fibres. Gels can be applied in topographies with special relief which can be cleaned without damaging, they can be reusable in some cases and they are safe for the conservators. However special attention should be given when enzymes or chemical solvents are used in gel’s composition. [3,4,14,15].Gels are soft materials consisting of interconnected long polymer chains dispersed in a fluid (water or/and organic solvents), forming a three-dimensional network. Hydrogels such us Agar, Agarose, Gellan gum, are water-based and may contain active substances: enzymes, chelators, emulsifiers and microemulsions (w/o) [7,16,17,18]. When the liquid portion is a solvent then gel is called Organogel. Gellan gum gel has been in use since 2003 as cleaning agent for paper artworks in Italy [19]. PMMA (Polymethyl Methacrylate, commonly called plexiglass) and Nevek which is a modified type of Agar gel, when mixed with green solvents: Diethyl Carbonate, Ethyl lactate or commonly used chemical solvents: Toluene, Ethanol, Acetone, form organogels [1,5,20].In this study, the following gel systems were used on the mock-up samples and the case-study object: Agar-enzyme lipase gel system for acrylic glue residue removal, Gellan gum loaded with Trizma base (pH regulator), Calcium acetate (gel regulator) and EDTA or DTPA for chelating activity to metal ions. As well as organogels PMMA or Nevek mixed with organic solvents: Diethyl Carbonate, a 75% biodegradable solvent and Petroleum ether for tape backing detachment from purpose made mock-ups.For the study’s needs a printed silk scarf was selected from the collections of the Historical and Ethnological Society of Greece of the National Historical Museum. Condition and pathology of the case-study object address the objectives of the study. The silk scarf depicts the "Temple of the Holy Trinity Greek Orthodox Church of Vienna" and measures 566mm width to 633mm length, Figure. 1. The design and inscriptions are printed on an off-white silk substrate with black ink, which was lined with a white fabric on a later date. The drawing was designed by Leopold Lieb and the engraving performed by Josef Jung [21]. Such printed scarves and etchings on paper were created from 1820 until 1857 to commemorate Sina’s family donations for the renovation of the church [22].
 | Figure 1. Silk scarf, depicting the temple of the Holy Trinity Greek orthodox church of Vienna. © National Historical Museum of Greece, import directory number: 11498 |
2. Materials and Methods
2.1. Photographic Documentation
- Τhe photographic documentation was performed in VIS light with symmetrical, raking and transmittance lighting. A Nikon D800 digital camera with an AF-S NIKKOR 24-70mm lens, 1:2.8 G ED (ø 77mm) and a tripod were used. The reflectance of the substrate and the fluorescence of the support under UV radiation at 365nm were recorded photographically with the same camera. On the camera lens the following filters: B+W MRC (UV-IR cut) N° 486 and 2E Kodak, were adjusted for the UVFC photography.
2.2. Hyper-Spectral Imaging
- For the observation and digital imaging of the case-study object a hyperspectral imaging system MU. SIS HS (ForthPhotonics) was used. Its spectrum sensitivity initiates at 400nm and reaches up to 1000nm. The software enables the acquisition of a spectral cube, which comprises images of a chosen area within the aforementioned space [24]. Snap-shots were taken from 420-1000nm, with a 20nm step. False colour infrared images and visible colour images were also acquired with the same system.For the photographic documentation see Appendix, Figures 1-6.
2.3. Fourier Transform Infrared Spectroscopy
- Spectroscopy FTIR spectra were carried out on a Perkin Elmer Spectrum GX FTIR spectrometer equipped with DTGS detector and a Pike MIRacle ATR ZnSe crystal (2mm sample diameter, 2,4 refractive index). A total of 20 scans per spectra were performed at 1cm−1 interval with 4.0cm-1 resolution. In special cases spectra were carried out on a Shimadzu FTIR-8300 spectrophotometer, where the above ATR system was adapted (64 scans per spectra, 4.0cm-1 resolution). Measurements were recorded between 4000 and 520 cm−1 in both cases.To determine the deposits and stains composition, the substrate spectra subtracted from the deposits or stains spectra. Spectra were processed with Perkin Elmer Spectrum software (v.5.3.1), Shimadzu FTIR 8000, and SpectaGryph 1.2.11 Spectroscopy software.
2.4. X-Ray Fluorescence Spectroscopy
- For the micro-XRF analyses a customized model of the ARTAX portable micro-XRF spectrometer ArTAX Bruker, Nano GmbH was utilized at the Institute of Nuclear and Particle Physics, National Centre for Scientific Research “Demokritos”, Athens.The spectrometer probe consists of an X-ray micro focus Rh-anode tube (spot size 0,008mm2, 50 kV (max), 0.6 mA, 30 W maximum power consumption with 0.2 mm Be window thickness) and a polycapillary X-ray lens as a focusing optical element that offers a focal distance of about 21mm and a spatial resolution of about 40 µm. The X-ray detection chain consists of a thermoelectrically cooled 10mm2 silicon drift detector (X-Flash, 1000B) coupled with a digital signal processor. A colour CCD camera attached to the spectrometer head in 45° angle can offer live documentary image of the analysed spot [25].Measurements were processed and converted into spectra by ArTAX and PyMca software. Spots (300sec), line scans (150sec/measurement, step 0,2 and 0,5mm) and area scans (300, 150, 50 sec/measurement, step 0,2 and 0,15mm) were performed on silk, printed areas and various stains (50keV, 600μA, unfiltered excitation, beam diameter 100μm).For comparable results, all measurements were reduced to 300sec/step. The average of representative measurements of the silk substrate was calculated. Τo obtain results related to the composition of stains and depositions, the average of silk measurements was subtracted from each measurement.
2.5. Optical Microscopy
- The observation and identification of the fibres was carried out on a LEICA DMLP, OLYMPUS Soft Imaging Solutions microscope, equipped with colour view camera (Soft Imaging System). Photos were taken with transmitted light and the use of polarizing filters in magnifications 10x, 20x, 40x.
2.6. SEM/EDS
- Sample from the silk (3mm2) and of the lining fabric (1cm2) were observed and photographed (magnification 30x-1000x) on a Scanning Electron Microscope-SEM JEOL JSM–6510LV, on low pressure vacuum 20Pa, and 20kV accelerating voltage. Elemental analysis of the samples was carried out with Energy dispersive X–ray spectroscopy (EDS), with a JEOL JSM 6510LV SEM, on low pressure vacuum 25Pa, 20kV accelerating voltage. The instrument is equipped with an X–act Pentafet Precision detector (INCA analysis system, Oxford Instruments).
2.7. Measurements
- The case-study object's conservation treatment was evaluated by measurements on specific areas before and after cleaning, by means of colorimetry (CIEL*a*b*), gloss (20°/60°/75°) and pH measurements. On every spot, three measurements were taken and the average was calculated.
2.7.1. Colorimetry
- For the colorimetry measurements a Lovibond RT Series reflectance tintometer was used according to the CIEL*a*b* model. The colour distance is calculated based on the following equation (1):
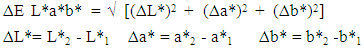 | (1) |
2.7.2. Gloss measurement
- For the gloss measurements a Novo-Gloss 20/60/75° Rhopoint Instruments gloss meter was used. The subtraction of final minus initial measurement value indicated gloss alterations of the materials due to accelerated ageing or cleaning treatments with gels (2):
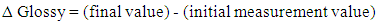 | (2) |
2.7.3. pH Measurement
- For the pH measurements, the inoLab pH 720 contact electrode pH Meter was used on mock-up’s surface which was previously wetted with one drop of deionized water. On special cases a piece of Agar gel 5% w/v (1cm2) was placed on specified areas of the case study object for 20 min. Then the surface of the gel which was in contact, was measured and the pH value was recorded at 24-25°C.
2.8. Mock-up’s Preparation
- Undyed silk fabric (Habutai 50g/m2, Tsiakiris Silk Industry, Soufli, Greece), was used for mock-ups preparation. Engraving was printed on the silk fabric with Charbonnel etching ink N° 55985, Noir Taille Douce, Charbonnel France. Prior to accelerated ageing the samples were cut into pieces measuring 5cm2 each and five categories of samples were created, so as to imitate the stains of the silk scarf:A. Tesa films (transparent universal 33m/15mm N°57341-00008 and N°57345-00004, Tesa, Germany) were sticked on printed silkB. Filmoplast P90 Neschen, Germany and 3M Scotch Magic tape 810, 3M USA were sticked on silkC. Dust and dirt deposits were applied on silkD. FeCl3 powder deposition was applied on silkE. Glucose stains (Glucose 1%w/v solution in deionized water) were applied on silk. During artificial ageing samples were secured with thread on a metallic rack.
2.9. Accelerated Aging
- The accelerated aging treatment of the mock ups was carried out on a Solar Climatic Chamber Test System ATLAS SC 340 MHG (Solar Simulator) equipped with irradiation system (MHG 1200-UV metal-halide lamp, 1200W), attached to the top of the test system. The beam path of the lamp is directed by a parabolic segment reflector system made of anodized aluminium [26]. Temperature and relative humidity conditions were stable for 37 days at 65°C, 55%RH. Aiming at simulating day-night sequences the mock-ups were simultaneously UV-irradiated periodically (cycle A: 3min UV irradiation/44min, 180 repeated cycles, followed by cycle B: 1,5min UV irradiation/44 min, 180 repeated cycles.
2.10. Gel-systems
2.10.1. Gellan Gum Gel System
- For the preparation of Gellan gum gel 2-4% w/v, Calcium acetate 0,4 g/L was dissolved in completely deionized water in a vial and then 2-4g of Gellan gum (KelcoGel CG-LA, CP Kelco, Atlanda, USA) was added depending on the desired concentration of the gel. The mixture was stirred and 0,2M Trizma buffer was added until the pH of the solution reached 8,5. Finally, completely deionized water up to 100ml was added. The vial with the mixture was placed in a microwave oven at 340watt for 30-60sec with intermediate stirring. When the viscous gel solution reached boiling point, it was poured into a flat square container to become gel (gel thickness ~50mm, pH: 7,5 [4].
2.10.2. Gellan Gum-Chelators Gel System
- A mixture of Gellan gum 2% w/v (CG-LA), Calcium acetate 0,4g/L, Trizma 0,2M and EDTA 1% w/v or DTPA 1% w/v was prepared in a vial (mixture pH: 6,6-7,2). The mixture was placed in a microwave oven at 340watt for 30-60sec with intermediate stirring. The viscous gel solution poured into a flat square container to become gel (gel pH: 6,2-6,9) [27,28].
2.10.3. Agar Gel
- For the preparation of Agar gel, 5g Agar powder (Fluka, gel temp. 35°C) was dispersed in deionized water. The mixture was stirred and 0,2M Trizma buffer was added until the pH of the solution reached 8,5. Deionised water was added up to 100ml. The mixture was heated to boiling (80°C, two cycles) in a microwave oven (600Watt, 2min) stirring occasionally [14,29].For the Agar-lipase gel system, a 2% w/v agar-deionized water mixture prepared and 0,2M Trizma buffer solution was added (mixture pH: 7,8). The mixture was heated to boiling in a micro-wave oven (two cycles). A solution of 0,1g lipase in 50ml water was added to the agar solution at 40°C on a magnetic stirrer (gel pH: 7,5) [3,6,18].
2.10.4. Organogels
- For the preparation of Nevek-DEC (Diethyl Carbonate, Sigma Aldrich) gel system, 20g of Nevek (25%w/v in water, pH: 3,35, CTS, Italy) was boiled in a microwave oven until melt. The vial was placed immediately on a magnetic stirrer and sealed with transparent membrane. In the vial with the melted Nevek, 5ml of Diethyl Carbonate was poured, while stirred. The mixture was poured into a petri dish and left to cool down. An amount of DEC solvent was observed on the surface of the gel. The gel system stored in a closed petri dish, as DEC is a very volatile solvent. The same procedure was followed for the preparation of Nevek-Petroleum ether gel system: [20g Nevek (25%w/v in water)-5ml Petroleum ether].For the preparation of PMMA-DEC 50%w/v gel system, 50g of Polymethyl Methacrylate (Sigma Aldrich) were put in a borosilicate vial and DEC was poured until 100ml. The sealed vial was placed on a magnetic stirrer and the mixture PMMA-DEC stirred for 3h, at 70°C till melt. The gel was used only for Tesa backing tape detachment from mock-ups [1].
3. Results and Discussion
3.1. Optical Microscopy, Infrared Spectroscopy, X-ray Fluorescence and SEM/EDS Analysis
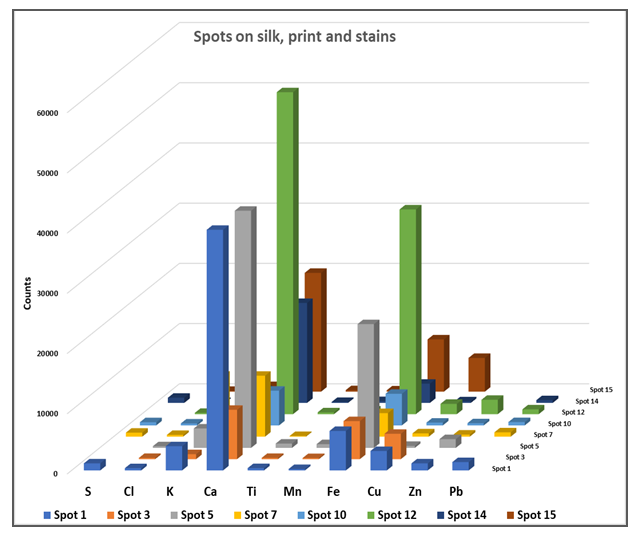
- Fibres of the object and the support fabric were examined and identified with optical microscopy and FTIR/ATR analysis. Object’s fibres had regular uniform shape, they were apparently featureless and identified as silk (Amide I 1619cm-1, Amide II 1517cm-1, Amide III 1226cm-1, Alanine 1440cm-1, Tyrosine 1161cm-1, N-H 3280cm-1 and C-H 2923 cm-1 stretch vibrations) [31]. The XRF analysis of the silk fabric showed high amount of Ca and traces of K, Fe, Cu and Pb. The SEM/EDS in addition with XRF fibre elemental analysis revealed that is a non-weighted silk. The metallic elements are deposits on the surface of the silk due to the printing procedure whereas Ca is due to the manufacture processes of the silk.The support fabric was identified as cellulosic material since longitudinal striations were evident on the surface of the fibres. The FTIR/ATR spectra gave the characteristic peaks of cellulose (3450-3100cm-1, 1363, 1312, 1259cm-1 and 1100-950cm-1 cellulose fingerprint). Due to the absence of C-OH stretching maxima of the glucose ring at 1100cm-1, the fabric was identified as viscose [32].Lining adhesive is pure paraffin wax with characteristic peaks at 2916, 1849, 1465 and 718cm-1 [32,33].Engraving ink was identified as a mixture of Carbon black (1563cm-1), Bone black (1099, 598cm-1), Calcium carbonate (1423, 873 cm-1), Nitrates (1317 cm-1), Silicates (1029 cm-1) and an oily binder (2921, 2846, 1717, 1460cm-1). XRF elemental analysis revealed the presence of Fe, Cu, Mn metal oxides. Ink composition match with the 19th century chalcographic inks, mentioned at Albertin’s and Romero’s studies. According to these studies the main elements of printing inks are K, Ca, Fe, Mn, Cu and Zn [34,35].Mock-up’s Charbonnel ink, “Noir Taille Douce”, (Charbonnel, France) was investigated by means of XRF elemental analysis. High amounts of Fe and Zn were traced. According to Manso study on Charbonnel ink, the main components are iron oxides and calcium compounds [36]. XRF elemental and FTIR/ATR molecular analysis gave information about silk degradation, characterization of stains, glue residues and deposits on the silk substrate, Figure. 2.
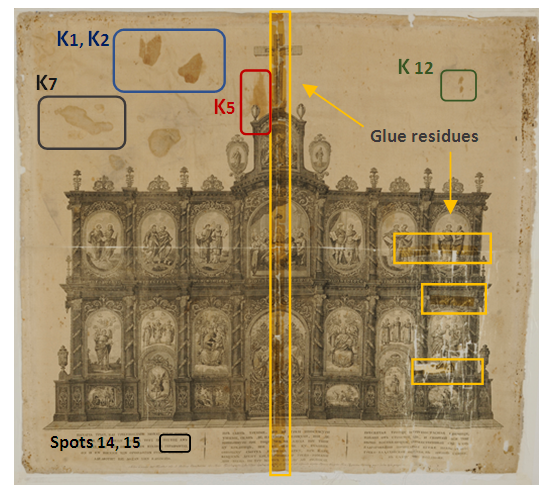 | Figure 2. Ink, stains and oxidized acrylic glue residues areas examined by means of XRF and FTIR/ATR spectroscopy. © National Historical Museum of Greece |
3.2. Comparative results of Chelating Gel Systems
- Gellan gum-chelators EDTA and DTPA gel systems were used on FeCl3 oxidized stain, at silk mock-ups (Gellan gum 2%w/v, EDTA 1%w/v, Calcium acetate 0,4g/L, Trizma 0,2M), (Gellan gum 2%w/v, DTPA 1%w/v, Calcium acetate 0,4g/L, Trizma 0,2M).Their effectiveness was studied in selected application times: every 10min, from 10 to 60min with evaluation criteria: colorimetry, gloss 20°/60°/75° and pH measurements on the FeCl3 stain area and gel surface. It was observed that after 20min of the EDTA gel system application, and after 10min of the DTPA gel system application, the ΔE colorimetry index decreased and the discoloration of the stain did not further improve. So, it was decided that beyond 30 minutes, applications should be made with the opposite side of the gel, not been used.In 10min application DTPA showed better performance compared to EDTA, however in 20min and 30 min application the action of EDTA continued to have a positive effect on stain’s discoloration. The optical result was documented by the high values of ΔΕ colorimetry index. In the interval between 30-60min (second application with a new gel piece) higher values of the ΔΕ colorimetry index were observed, Figure. 4.
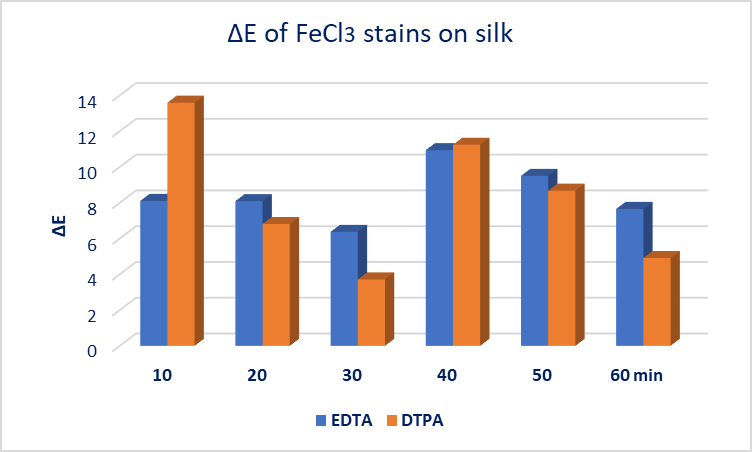 | Figure 4. EDTA 1%w/v and DTPA 1%w/v Gellan gum gel system application results on oxidized FeCl3 stain on silk |
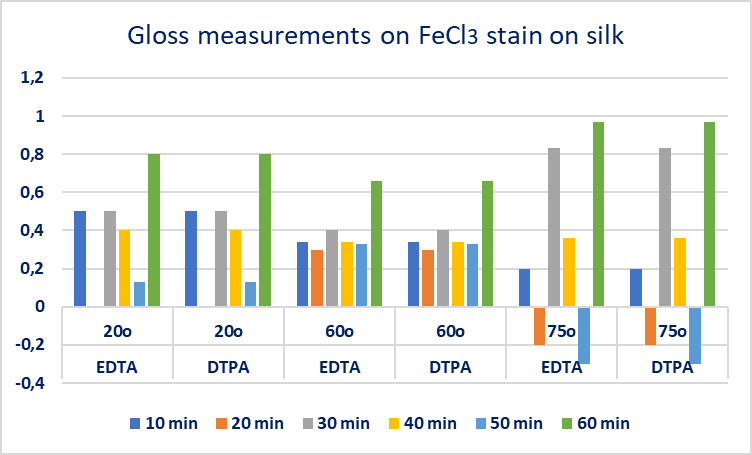 | Figure 5. Gloss measurements 20°/60°/75°, on FeCl3 oxidized stain on silk treated with EDTA 1%w/v and DTPA 1%w/v Gellan gum gel system |
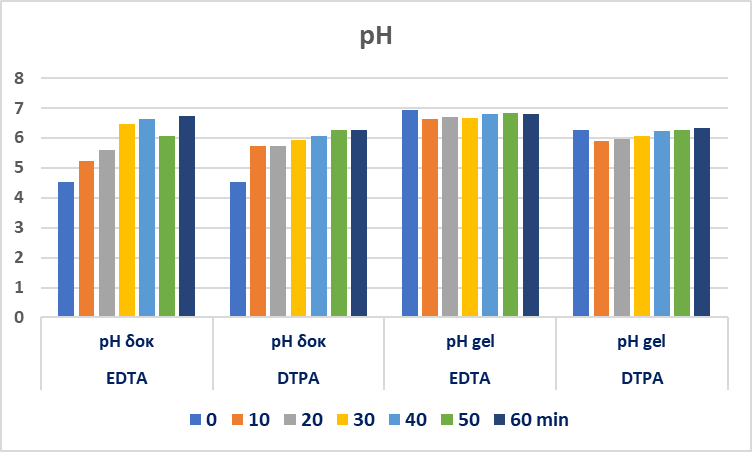 | Figure 6. pH measurements on the EDTA and DTPA gel system application areas and the gel’s application surface |
3.3. Results of Gel Application
- Documentation and chemical composition analysis of the material to be removed enable the development of an optimal gel system design and the selection of the appropriate gel systems for stains, depositions, tidelines, and oxidized acrylic glue residues.On polysaccharide stains, a piece of hydrogels Agar 4%-5%w/v and Gellan gum 2%w/v gel system (Gellan gum, Calcium acetate 0,4g/L and Trizma 0,2M) were placed on the stains for 10min. After removing the gel pads from the surface, a pale yellow to brown discolouration on most gel pads could be observed by visual inspection. Concerning the water-soluble stains removal, as the hydrogel concentration increases, the diffusion effect is reduced and the action time increases from 10 to 20min. Repetitive applications with new piece of gel led to successful stain removal, Figures. 7, 8.
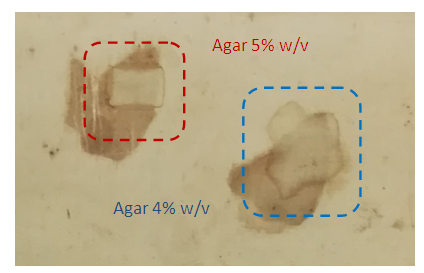 | Figure 7. Application results of the polysaccharide stain cleaning from silk, with Agar 5%w/v and Agar 4%w/v |
 | Figure 8. Application results of the polysaccharide stain cleaning from silk, with Gellan gum 2%w/v, Calcium acetate 0,4g/L and Trizma 0,2M gel system |
 | Figure 9. Agar-lipase gel system application on mock-ups verso side to activate tape adhesive glue |
 | Figure 10. Silk scarf after cleaning treatments with gels |
 | Figure 11. Agar 2%w/v-enzyme lipase gel system application on the oxidized glue residue deposition |
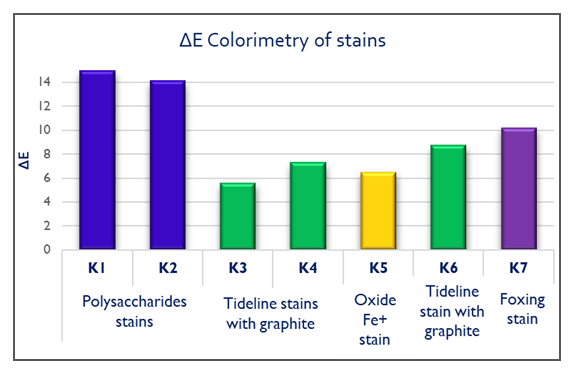 | Figure 12. ΔΕ Colorimetry results of stains after gel treatments |
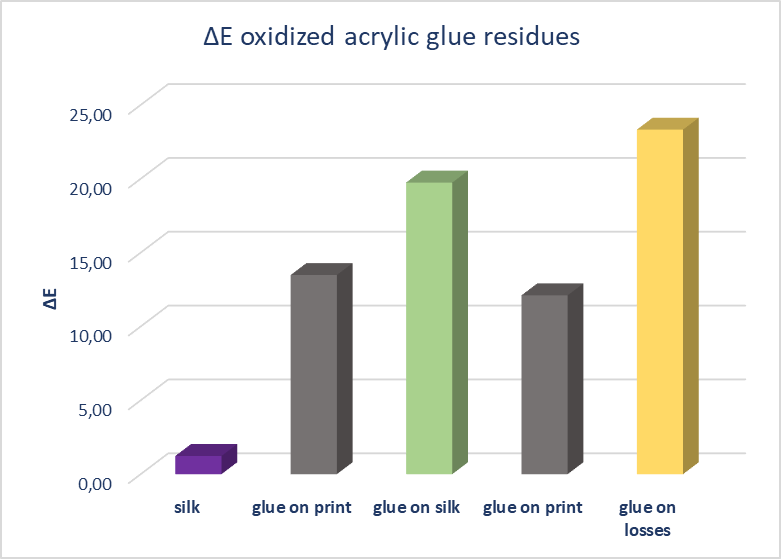 | Figure 13. ΔΕ Colorimetry results of oxidized acrylic glue residue on silk, print and support areas, after gel treatments |
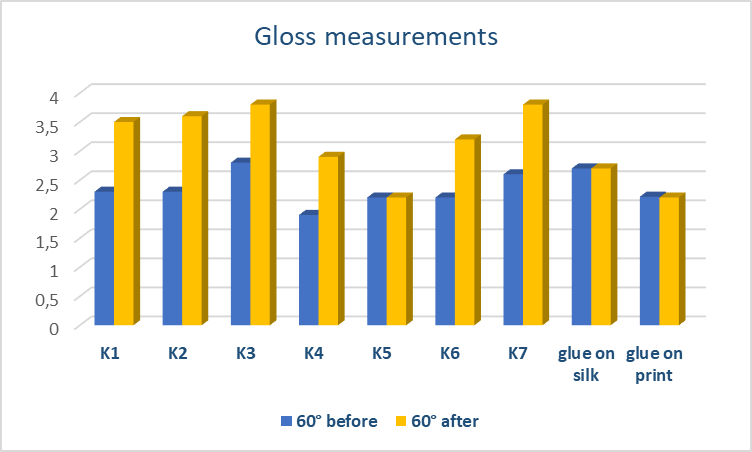 | Figure 14. Gloss measurements (60°) on stains and glue residue areas before and after cleaning |
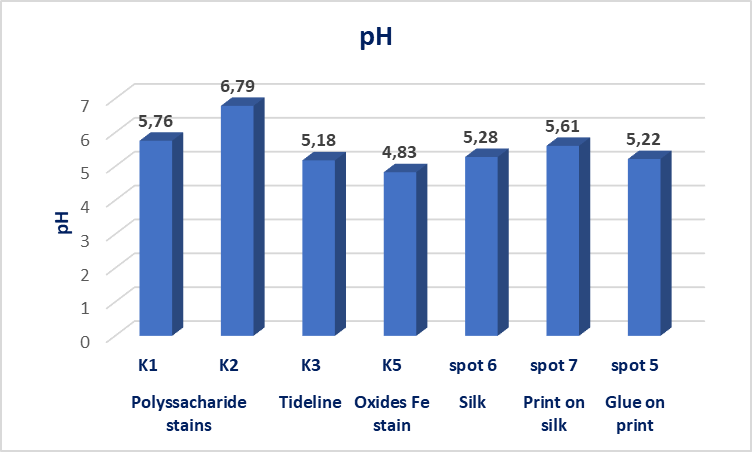 | Figure 15. PH measurements on stains, silk, print on silk and glue residue areas before cleaning |
3.4. Conservation Treatment
- For the selective removal of stains on the silk scarf a piece of Tengujo Japanese paper, weighing 4g was placed on the surface to be cleaned for minimising gel residue deposition. Then a piece of pre-cut gel with the stain shape was placed onto the Japanese paper for approximately 10min. Melinex and a light weight of plexiglass covered the gel. Repeated applications reduced or totally removed the stains. Agar-enzyme lipase gel system applications, for the acrylic glue residue removal, took place on a heated table at 40°C. Repeated applications removed most of the oxidized acrylic glue residue. Then a piece of agar gel 5%w/v was placed on stained areas to remove enzymes residues. The silk substrate was finally treated on a suction table with deionized water to remove tideline stains after gel cleaning applications. After the old support was removed, the silk scarf was supported with a silk crepline fabric prepared with an adhesive on a heated table, at 60°C, in vacuum. The silk crepline was previously prepared with 12%w/v adhesive-water solution (Acrylkleber 360HV/4001 and Acrylkleber 498HV/ 4005 Lascaux, in a ratio 1 to 2) and allowed to dry for 12 hours.For the construction of the reinforced mounting panel, acid-free foamboard type cardboard was covered with soft cotton fabric (flannel) and silk Habutai fabric, dyed in similar colour with the object. The object was attached to the display panel with stitches [41]. Finally, the mounted silk scarf was placed on an acid-free cardboard box, Figure. 16.
 | Figure 16. The silk scarf mounted on the reinforced panel |
3.5. UVFC Imaging
- At the UVF colour photographic documentation, the carbon black engraving ink do not fluoresce and appear dark. On the contrary, the cellulosic support presents an intense fluorescence due to bleaching process of the fabric. The fibres observation on SEM confirmed the hypothesis, Figure. 17.
 | Figure 17. UVFC photographic documentation (365nm), of the silk scarf before conservation © National Historical Museum of Greece |
 | Figure 18. UVFC photographic documentation of the silk scarf after gel treatments © National Historical Museum of Greece |
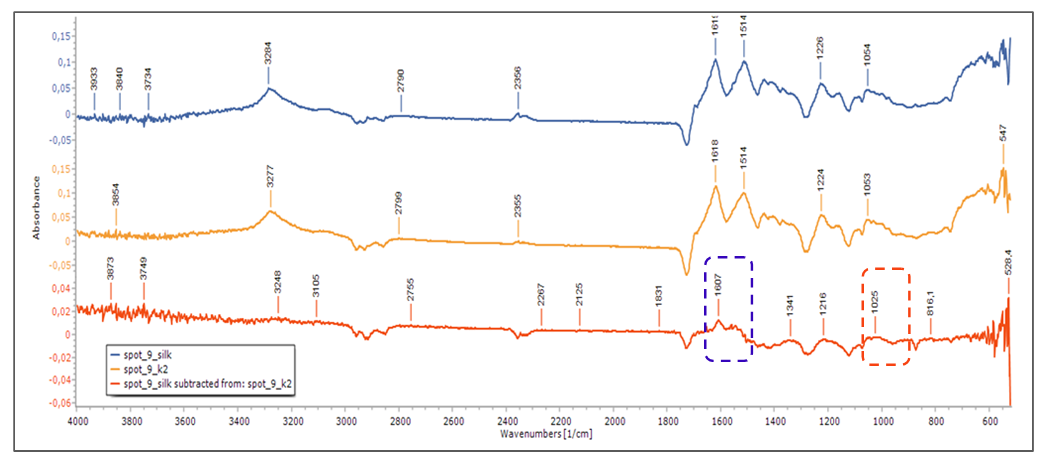
3.6. The Residue Issues
- FTIR/ATR spots subtracted spectra gave answers about the gel’s residue issue. Each spot spectrum after gel treatment subtracted from the corresponding spot spectra before cleaning. No deposits or traces of Agar gel were detected, except for calcium acetate trace residues (1607 and 1025cm-1), which was used as Gellan gum’s viscosity regulator, Figure. 19.
4. Conclusions
- The study results of the gel applications on mock-up’s enable the selection of the appropriate gel systems for the selective cleaning of the printed scarf, minimising risk and treatment duration.During the cleaning applications on mock-ups and the case-study object, the action of gel systems controlled on a regular basis using objective methods of measurement. This allowed to determine the optimal duration and efficiency of gel’s applications.The removal of water-soluble oxidation from the fabric substrate improved its long-term durability while the reinforcement of the silk substrate with a new support fabric increased its mechanical strength and restored its flat form. The effectiveness of cleaning applications was considered successful according to the properties of the selected gel systems, the methods that were followed and the available consumables.Conservation treatments contributed to the improvement of the object’s aesthetic and preservation condition. The artefact can now be on display at periodic exhibitions of the museum.Through gel treatments targeted and selective action of cleaning can be achieved. Critical factors for gel’s effectiveness are pH, gel’s viscosity and active substance’s concentration on gel system. For successful results gel systems should be designed, depending on the chemical composition of the material to be removed and the preservation state of the object. As silk is a hydrophilic material, high concentrations (5% w/v) should be used, to avoid diffusion effect.In general, gels are a safe method for silk conservation.
ACKNOWLEDGEMENTS
- I would like to thank the council of the Historical and Ethnological Society of Greece for giving me the permission to study and to conserve the case-study object from the National Historical museum’s collections.I am grateful, to the supervisor of my post-graduate thesis Dr Stamatis Boyatzis and the members of the committee Dr Anna Karatzani and Dr Andreas G. Karydas for the scientific oversight and contribution to the successful completion of this study. I also wish to thank Dr Agathi-Anthoula Kaminari for being so helpful with non-destructive photographic documentation techniques, Dr Stavroula Rapti for the SEM/EDS analysis and ARTICON lab, Dept. of Conservation of Antiquities and Works of Art, University of West Attica, for the facilities that were used.
Appendix
 | Figure 1. Silk scarf before cleaning treatment with gels © National Historical Museum of Greece |
 | Figure 2. Silk scarf after cleaning treatment with gels © National Historical Museum of Greece |
 | Figure 3. Silk scarf photographed with raking light, before conservation © National Historical Museum of Greece |
 | Figure 4. Silk scarf photographed with transmitted light photography, before conservation © National Historical Museum of Greece |
 | Figure 5. IR False Color Imaging (FCIR), MuSIS Hyperspectral Imaging © National Historical Museum of Greece |
 | Figure 6. Hyperspectral Imaging at 820nm © National Historical Museum of Greece |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML