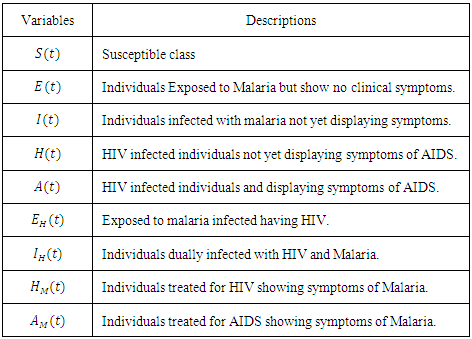
-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Applied Mathematics
p-ISSN: 2163-1409 e-ISSN: 2163-1425
2016; 6(3): 56-63
doi:10.5923/j.am.20160603.03

Mathematical Analysis to Reduce the Death Rate of HIV –Malaria Co-infection
Udoy S. Basak 1, Bimal Kumar Datta 2
1Lecturer, Department of Mathematics, Pabna University of Science & Technology, Pabna, Bangladesh
2Assistant Professor, Department of Mathematics, Pabna University of Science & Technology, Pabna, Bangladesh
Correspondence to: Udoy S. Basak , Lecturer, Department of Mathematics, Pabna University of Science & Technology, Pabna, Bangladesh.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

HIV/AIDS and Malaria are the two great threats for human being. These are causing a lot of death every year. Here comprehensive mathematical techniques have been used to analyze the co-infection of HIV-Malaria. A mathematical model of co-infection has been formulated. It is found that, using the next generation matrices, the disease free equilibrium point is locally asymptotically stable when the reproduction number is less than unity and unstable when reproduction number is greater than unity. Centre manifold theory is used to show that the HIV/AIDS-malaria co-infection model`s endemic equilibrium point is locally asymptotically stable when the associated reproduction numbers are less than unity. It has shown that, reduction of sexual activities among the HIV infected population will reduces the HIV/AIDS in the society. As well as it will also reduce the mortality rate of HIV- malaria co-infection.
Keywords: HIV, Malaria, Co-infection, Stability, Positivity
Cite this paper: Udoy S. Basak , Bimal Kumar Datta , Mathematical Analysis to Reduce the Death Rate of HIV –Malaria Co-infection, Applied Mathematics, Vol. 6 No. 3, 2016, pp. 56-63. doi: 10.5923/j.am.20160603.03.
Article Outline
1. Introduction
- Human Immunodeficiency Virus (HIV) is a virus that gradually attacks the immune system, which is our body’s natural defense against illness. If a person becomes infected with HIV, they will find it harder to fight off infections and diseases. The virus destroys a type of white blood cell called a T-helper cell and makes copies of itself inside them. T-helper cells are also referred to as CD4 cells [1, 4, 7].The first cases were reported in 1981 and today there are approximately 36.9 million people currently living with HIV and tens of millions of people have died of AIDS-related causes since the beginning of the epidemic [2, 3, 5]. While new cases have been reported in all regions of the world, approximately 70% are in sub-Saharan Africa [9]. Most people living with HIV or at risk for HIV do not have access to prevention, care, and treatment, and there is still no cure [15, 17]. HIV primarily affects those in their most productive years; about 38% of new infections are among those under age 25 [6, 8].HIV is found in the blood, sexual and bodily fluids of an infected person. The most common form of transmission are unsafe sex, unsafe blood transfusion, transmission from mother to child, intravenous drug use with contaminated needles and other blood related modes like bleeding wounds.It targets and infects white blood cells known as CD4+ T-cells which are part of the immune system. CD4+ T-cells are helper cells produced from precursors in bone marrow and thymus. When a person has chronic HIV infection, it causes gradual depletion of the CD+4 T-cell pool In a normal human being the level of CD+4 T-cells in the peripheral blood is regulated at a level between 800mm-3 to 1200mm-3 when the CD+4 T-cell count falls below 200mm-3, a person becomes vulnerable to opportunistic infections. CD+4 T-cells are a primary indicator used to measure progression of HIV infection in an HIV infected person [10, 11, 12]. Despite these challenges, new global efforts have been mounted to address the epidemic, particularly in the last decade, and there are signs that the epidemic may be changing course. The number of people newly infected with HIV and the number of AIDS-related deaths have declined, contributing to the stabilization of the epidemic. In addition, the number of people with HIV receiving treatment has increased to 15.8 million as of June 2015, a 2.2 million increase since June 2014. Young people, ages 15-24, account for approximately 30% of new HIV infections (among those 15 and over) [9, 13, 16].Malaria is an infectious disease caused by Plasmodium parasites and transmitted between humans through bites of female Anopheles mosquitoes. Malaria was first discovered centuries ago by the Chinese in 2700 BC [6]. However it was in the 1800’s when Ross made his ground breaking discoveries that led to our understanding of the mechanics behind malaria infections. About 3.2 billion people – nearly half of the world's population – are at risk of malaria. In 2015, there were roughly 214 million malaria cases and an estimated 438000 malaria deaths. Increased prevention and control measures have led to a 60% reduction in malaria mortality rates globally since 2000. Sub-Saharan Africa continues to carry a disproportionately high share of the global malaria burden. In 2015, the region was home to 89% of malaria cases and 91% of malaria deaths [8, 14].The most common first symptoms of malaria are similar to those of Flu. The patient may experience headache, aching muscles, stomach ache and weak or lack of energy. After a day or so the body temperature may rise (up to 40°C) and the patient may have fever, shivers, severe headache, diarrhea, loss of appetite, nausea, vomiting, back pain and increased sweating. The individuals most vulnerable to malaria are children under the age of 5 years. This is attributed to their weaker immunity. Aside from children, pregnant women are also heavily affected, with resultant effects on maternal health and birth outcomes.Treatment of malaria depends on many factors including disease severity, the species of malaria parasite causing the infection and the part of the world in which the infection was acquired. The latter 2 characteristics help determine the probability that the organism is resistant to certain anti-malarial drugs. Additional factors such as age, weight, and pregnancy status may limit the available options for malaria treatment [10].Malaria and human immunodeficiency virus (HIV) are the most deadly and important global health problems of our time. Malaria accounts for more than a million deaths each year, of which about 90% occur in tropical Africa, where malaria is the leading cause of mortality in children below five years [26]. Sub-Saharan Africa is also home to more than 29 million people living with HIV/AIDS. Both Malaria and HIV are considered as diseases of poverty, since they hinder sustainable development and contribute to poverty by taking a great toll on the young productive generation who would otherwise enter the workforce and contribute to the nation’s economy. The global distribution of Malaria and HIV is the same, with the majority of those affected living in sub-Saharan Africa, the Indian subcontinent, and Southeast Asia. Owing to the overlap of their geographic distribution and resultant rates of co-infection, interactions between the two diseases pose major public health problems [8].This study was carried out from October to November 2013 in Owena, Idanre Local Government Area and State Hospital Akure, Ondo State. A total of 150 people of different age groups and sexes were screened for malaria and co-infection with HIV. In Owena and State hospital, 88 and 62 blood samples were examined respectively for the presence of malaria using thick and thin smear, and also for the presence of Human Immunodeficiency Virus (HIV) using Abbott Determine kit and Stat Pak kit. A standardized questionnaire was used to obtain information’s from each person. Such information is those relating the infection rate with age, sex and state of origin, place of residence [19].
2. Formulation of Model
- In modelling the total human population at any time t, denoted by
 is subdivided into sub-population namely, Susceptible
is subdivided into sub-population namely, Susceptible  who are not yet infected either by HIV or malaria, exposed to Malaria but show no clinical symptoms of the disease
who are not yet infected either by HIV or malaria, exposed to Malaria but show no clinical symptoms of the disease 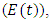 exposed to malaria infected having HIV
exposed to malaria infected having HIV 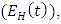 individuals infected with malaria not yet displaying symptoms
individuals infected with malaria not yet displaying symptoms 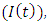 HIV infected individuals not yet displaying symptoms of AIDS
HIV infected individuals not yet displaying symptoms of AIDS 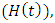 HIV infected individuals yet displaying symptoms of AIDS
HIV infected individuals yet displaying symptoms of AIDS 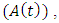 individuals dually infected with HIV and malaria
individuals dually infected with HIV and malaria 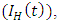 individuals treated for HIV showing symptoms of malaria
individuals treated for HIV showing symptoms of malaria 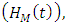 individuals treated for AIDS showing symptoms of malaria
individuals treated for AIDS showing symptoms of malaria 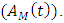 Thus we have
Thus we have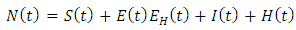
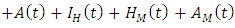 The susceptible population is increased by the recruitment of individuals (assumed susceptible) into the population at a rate
The susceptible population is increased by the recruitment of individuals (assumed susceptible) into the population at a rate  Susceptible individuals acquire Malaria infection, following effective contact with the people infected with Malaria only (i.e. those in the E,
Susceptible individuals acquire Malaria infection, following effective contact with the people infected with Malaria only (i.e. those in the E,  and
and 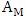 classes) at a rate
classes) at a rate 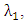 where
where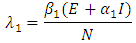
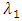 is the force of infection for malaria. Here
is the force of infection for malaria. Here 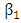 is the transmission rate for Malaria and
is the transmission rate for Malaria and 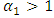 accounts for the assumed reduction of infectivity of infectious individuals in the exposed class and known as the modification parameter.Similarly, the susceptible individuals acquire HIV from with HIV at a rate
accounts for the assumed reduction of infectivity of infectious individuals in the exposed class and known as the modification parameter.Similarly, the susceptible individuals acquire HIV from with HIV at a rate 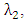 where
where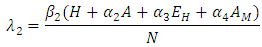
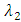 is the force of infection for HIV. Here
is the force of infection for HIV. Here 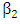 is the transmission rate for HIV and
is the transmission rate for HIV and 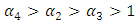 accounts for the assumed reduction of infectivity of infectious individuals in the exposed class and known as the modification parameter.In the susceptible class, population is entering into this class at a constant rate
accounts for the assumed reduction of infectivity of infectious individuals in the exposed class and known as the modification parameter.In the susceptible class, population is entering into this class at a constant rate  In the malaria exposed class
In the malaria exposed class 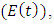 individuals entering into this class from susceptible class
individuals entering into this class from susceptible class 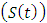 at a rate
at a rate 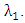 Also individuals entering into the HIV class
Also individuals entering into the HIV class 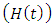 from susceptible class
from susceptible class 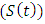 at a rate
at a rate 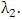 In the malaria infected class
In the malaria infected class 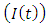 , individual progresses to Malaria infected class
, individual progresses to Malaria infected class 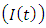 from the Malaria exposed class
from the Malaria exposed class 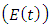 at a rate
at a rate 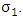 In this class, let
In this class, let 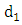 denotes the death rate due to the disease. In AIDS class
denotes the death rate due to the disease. In AIDS class 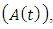 individual is advancing to AIDS class
individual is advancing to AIDS class 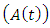 from HIV infected class
from HIV infected class 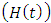 at a rate
at a rate 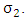 In this class, let
In this class, let 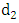 denotes the death rate due to the disease.In the exposed to malaria infected having HIV class
denotes the death rate due to the disease.In the exposed to malaria infected having HIV class 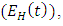 individuals forward movement rate to malaria infected having HIV class
individuals forward movement rate to malaria infected having HIV class 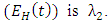 Dually infected with HIV and malaria class is denoted by
Dually infected with HIV and malaria class is denoted by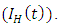 Now the individuals progress to dually infected with HIV and malaria class
Now the individuals progress to dually infected with HIV and malaria class 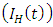 from the exposed to malaria infected having HIV class
from the exposed to malaria infected having HIV class 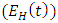 at a rate
at a rate 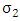 . Also individually progress to this class from Malaria infected class
. Also individually progress to this class from Malaria infected class 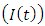 . In this class, let
. In this class, let 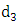 denotes the death rate due to the disease.HIV class having Malaria is denoted by
denotes the death rate due to the disease.HIV class having Malaria is denoted by 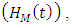 individual’s forward movement to HIV class having Malaria
individual’s forward movement to HIV class having Malaria 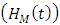 from the HIV class
from the HIV class 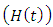 at a rate
at a rate 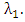 AIDS class having Malaria
AIDS class having Malaria 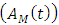 , individuals advancement to AIDS class having Malaria
, individuals advancement to AIDS class having Malaria 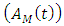 from the HIV class having Malaria
from the HIV class having Malaria 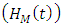 at a rate
at a rate 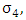 also individually progress to this class from AIDS class
also individually progress to this class from AIDS class 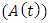 at a rate
at a rate 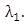 In this class, let
In this class, let 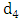 denotes the death rate due to the disease.In Malaria infected class
denotes the death rate due to the disease.In Malaria infected class 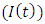 , AIDS class
, AIDS class 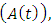 dually infected with HIV and malaria class
dually infected with HIV and malaria class 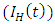 and AIDS class having Malaria
and AIDS class having Malaria 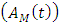 , the disease induced death rates are denoted by
, the disease induced death rates are denoted by 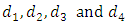 respectively. Further, natural mortality occurs in all classes is denoted by
respectively. Further, natural mortality occurs in all classes is denoted by 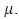 Combining all the aforementioned assumption and definitions, the model becomes:
Combining all the aforementioned assumption and definitions, the model becomes: Schematically this can be shown as,
Schematically this can be shown as,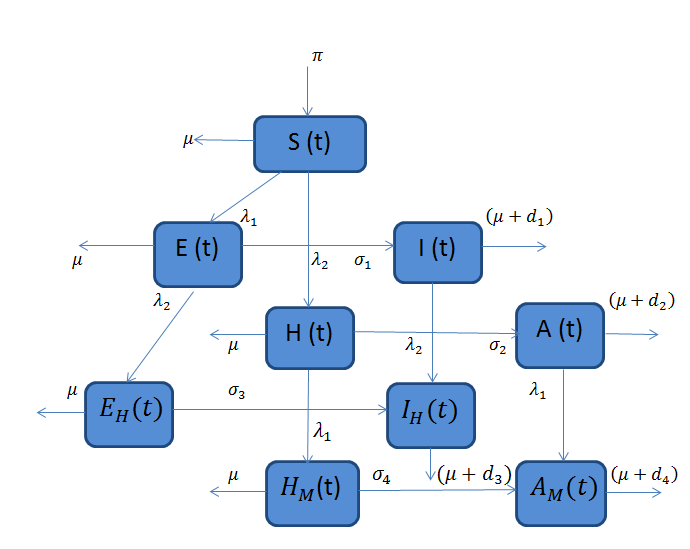 | Figure 1. Diagram of the model |
3. Positivity and Boundedness of Solution
- Model (1) describes the co-infection of Malaria and HIV diseases and therefore it can be shown that the associated state variables are non-negative for all time
 and that the solutions of the model (1) with positive initial data remains positive for all time
and that the solutions of the model (1) with positive initial data remains positive for all time 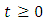 [15]. We assume the associated parameters are non-negative all time
[15]. We assume the associated parameters are non-negative all time 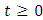 all feasible solutions are uniformly bounded in a proper subset
all feasible solutions are uniformly bounded in a proper subset 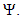 Theorem: Solutions of the model (1) are contained in a region where
Theorem: Solutions of the model (1) are contained in a region where 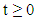 [15].Proof: To show that all feasible solutions are uniformly-bounded in a proper subset
[15].Proof: To show that all feasible solutions are uniformly-bounded in a proper subset 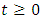 . Let
. Let 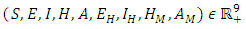 be any solution with non-negative initial conditions. From the theorem on differential inequality if t follows that,
be any solution with non-negative initial conditions. From the theorem on differential inequality if t follows that,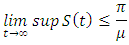 Taking the time derivative of N along a solution path of the model (1) gives
Taking the time derivative of N along a solution path of the model (1) gives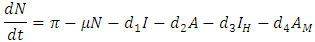 Then,
Then,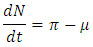 From the theorem on differential inequality it follows that,
From the theorem on differential inequality it follows that,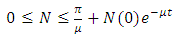 Where,
Where, 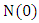 represents the value of (1) evaluated at the initial values of the respective variables. Thus as
represents the value of (1) evaluated at the initial values of the respective variables. Thus as 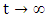 we have
we have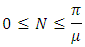 It follows that
It follows that 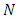 is bounded and all the feasible solutions of the model (1) starting in the region
is bounded and all the feasible solutions of the model (1) starting in the region 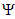 for all
for all 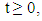 approaches, enter or stay in the region, where
approaches, enter or stay in the region, where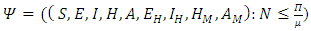 Thus Ψ is positively invariant under the flow induced by (1). Existence, uniqueness and continuation results also hold for the model (1) in
Thus Ψ is positively invariant under the flow induced by (1). Existence, uniqueness and continuation results also hold for the model (1) in 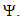 Hence model (1) is well-posed mathematically and epidemiologically and it is sufficient to consider its solutions in
Hence model (1) is well-posed mathematically and epidemiologically and it is sufficient to consider its solutions in 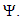
4. Disease-free Equilibrium Point
- Disease-free equilibrium (DFE) points of a disease model are its steady-state solutions in the absence of infection or disease. We denote this point by
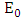 and define the “diseased” classes that are either exposed or infectious. Then can construct the following two lemmas. Lemma: For all equilibrium points on
and define the “diseased” classes that are either exposed or infectious. Then can construct the following two lemmas. Lemma: For all equilibrium points on 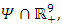 for which
for which 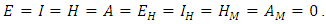 Then the positive DFE for the model (1) is
Then the positive DFE for the model (1) is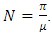 Lemma: The model (1) has exactly one DFE and the DFE point is
Lemma: The model (1) has exactly one DFE and the DFE point is 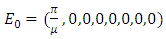 Proof: The proof of the lemma requires that we show that the DFE is the only equilibrium point of (1) on
Proof: The proof of the lemma requires that we show that the DFE is the only equilibrium point of (1) on 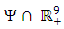 Substituting
Substituting 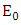 into (1) shows all derivatives equal to zero, hence DFE is an equilibrium point. From above lemma, the only equilibrium point for N is
into (1) shows all derivatives equal to zero, hence DFE is an equilibrium point. From above lemma, the only equilibrium point for N is 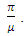 Thus the only equilibrium point for
Thus the only equilibrium point for 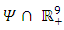 is DFE.
is DFE.5. Local Stability of the Disease-free Equilibrium
- The global stability of the model (1) is highly dependent on the basic reproduction number which is denoted by
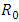 [3]. The basic reproduction number is defined as the expected number of secondary infections produced by an index case in a completely susceptible population. The associated nonnegative matrix F, for the new infection terms, and the non-singular M-matrix, V, for the remaining transfer terms are given respectively, by
[3]. The basic reproduction number is defined as the expected number of secondary infections produced by an index case in a completely susceptible population. The associated nonnegative matrix F, for the new infection terms, and the non-singular M-matrix, V, for the remaining transfer terms are given respectively, by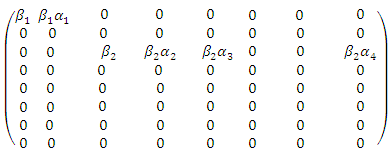 and
and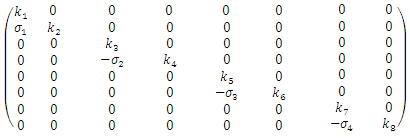 Where
Where 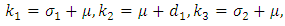
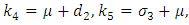
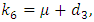
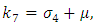
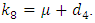 The basic reproduction number
The basic reproduction number 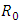 is the spectral radius of the
is the spectral radius of the 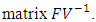 By using Maple the eigenvalues of the
By using Maple the eigenvalues of the 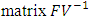 are
are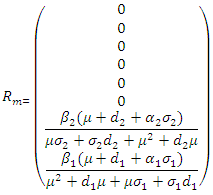
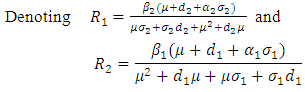 we have,
we have, 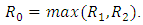 Thus we have the following lemmaLemma: The disease-free equilibrium
Thus we have the following lemmaLemma: The disease-free equilibrium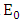 of the model (1) is locally asymptotically stable whenever
of the model (1) is locally asymptotically stable whenever 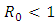 and unstable
and unstable 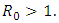
6. Global Stability of the Disease-free Equilibrium
- The global asymptotically stability (GAS) of the disease-free state of the model is investigated using the theorem by Castillo-Chavez. We rewrite the model as:
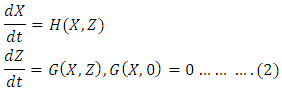 Where
Where 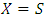 and
and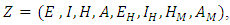 with the components of
with the components of 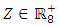 denoting the infected population.The disease free equilibrium is now denoted as:
denoting the infected population.The disease free equilibrium is now denoted as: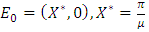 The condition must be met to guarantee a local asymptotic stability
The condition must be met to guarantee a local asymptotic stability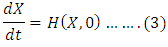 Here,
Here, 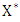 is globally asymptotically stable (GAS)
is globally asymptotically stable (GAS)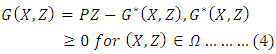 Where
Where 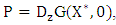 is an M-matrix (the off-diagonal elements of P are non-negative) and
is an M-matrix (the off-diagonal elements of P are non-negative) and 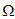 is the region where the model makes biological sense. If the system (3) satisfies the conditions of (4) then the theorem below holds:Theorem: The fixed point
is the region where the model makes biological sense. If the system (3) satisfies the conditions of (4) then the theorem below holds:Theorem: The fixed point 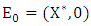 is a globally asymptotically stable equilibrium of the system (3) provided that
is a globally asymptotically stable equilibrium of the system (3) provided that 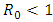 and the assumptions in (4) are satisfied.Proof: Form the model system (1) and (4), we have
and the assumptions in (4) are satisfied.Proof: Form the model system (1) and (4), we have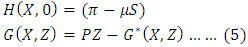 Where
Where 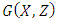 is as follows:
is as follows: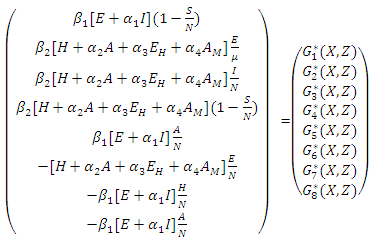 Here
Here 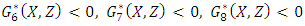 and so the conditions are not met. So
and so the conditions are not met. So 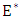 may not be globally asymptotically stable when
may not be globally asymptotically stable when 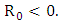
7. Endemic Equilibrium of the Model
- A disease is endemic in a population if it persists in a population. The endemic equilibrium of the model is studied using the Central Manifold Theorem [1]. To apply this theorem we make the following change of variables. Let
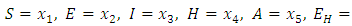
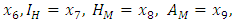 so that
so that 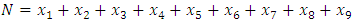 The model (1) can be rewritten in the form:
The model (1) can be rewritten in the form: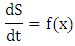 Where
Where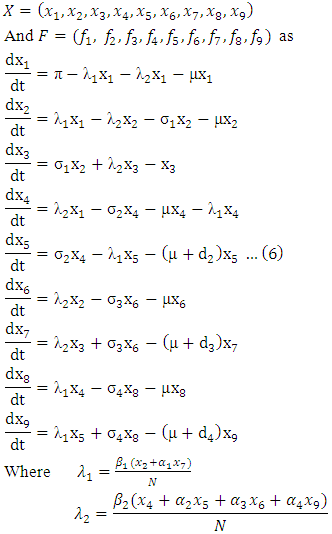 The Jacobian of the system (6) is
The Jacobian of the system (6) is 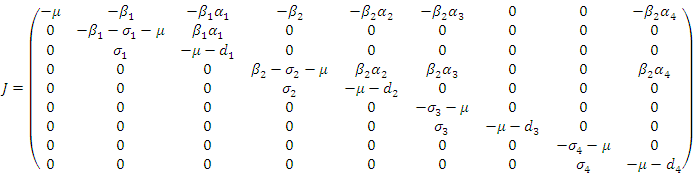 To analyze the dynamics of (6), we compute the eigenvalues of the Jacobean of (6) at the disease free equilibrium (DFE). It can be shown that this Jacobean has a right eigenvector given by:
To analyze the dynamics of (6), we compute the eigenvalues of the Jacobean of (6) at the disease free equilibrium (DFE). It can be shown that this Jacobean has a right eigenvector given by: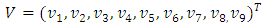 Where
Where 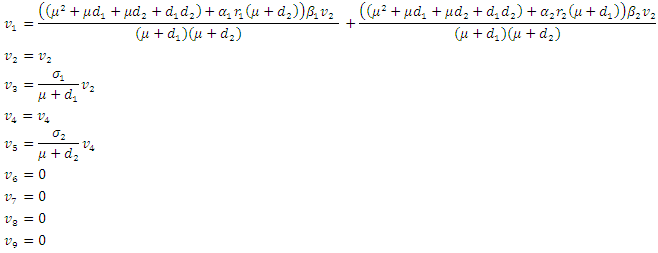 And the left eigenvectors are given by
And the left eigenvectors are given by 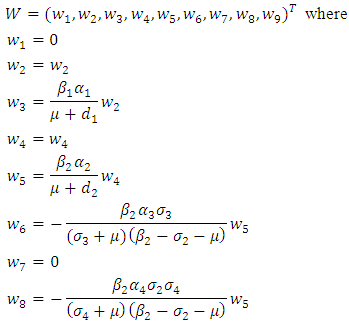 Now using (6) we have
Now using (6) we have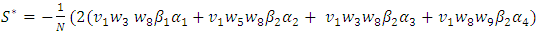 And
And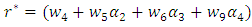 Clearly we can see that
Clearly we can see that 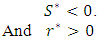 Thus we have established the following theorem:Theorem. The model (1) has a unique endemic equilibrium which is locally asymptotically stable when
Thus we have established the following theorem:Theorem. The model (1) has a unique endemic equilibrium which is locally asymptotically stable when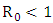 and unstable when
and unstable when 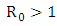 [16].
[16].8. Numerical Simulations and Discussions
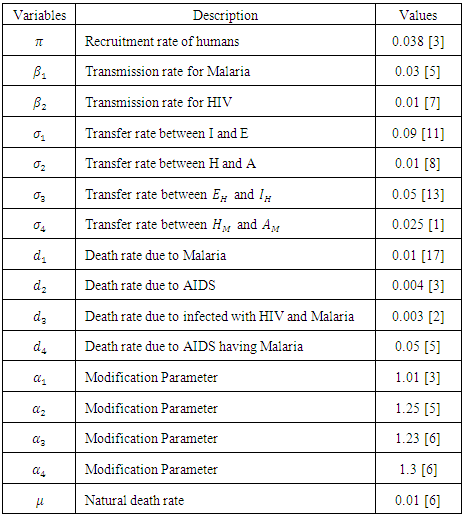
- The parameters and their values that are used the model are listed below.
|
|
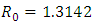 which is bigger than 1 and figure (3) shows that the infected population is decreasing when
which is bigger than 1 and figure (3) shows that the infected population is decreasing when 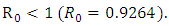 Figure (4) (prevalence of HIV infection) indicates that the number of total infected population increases whenever the basic reproduction number
Figure (4) (prevalence of HIV infection) indicates that the number of total infected population increases whenever the basic reproduction number 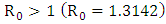 and the figure (5) shows that the prevalence of HIV infection decreases when
and the figure (5) shows that the prevalence of HIV infection decreases when 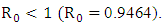 Figure (6) and (7) (HIV and Malaria co-infection) both indicate respectively that when the basic reproduction number
Figure (6) and (7) (HIV and Malaria co-infection) both indicate respectively that when the basic reproduction number 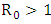
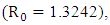 the number of total infected population increases and when
the number of total infected population increases and when 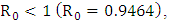 the number of total infected population decreases.
the number of total infected population decreases.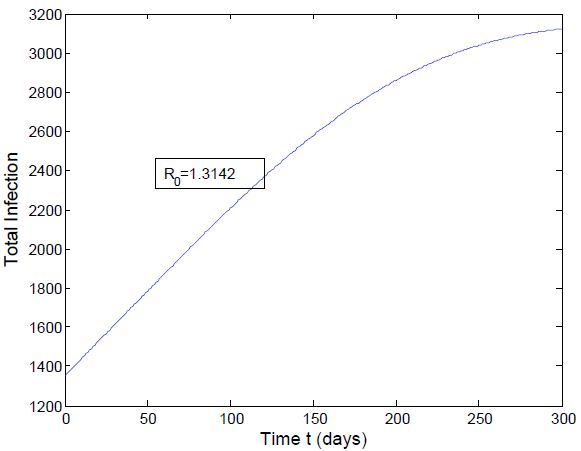 | Figure 2. Total infection when  |
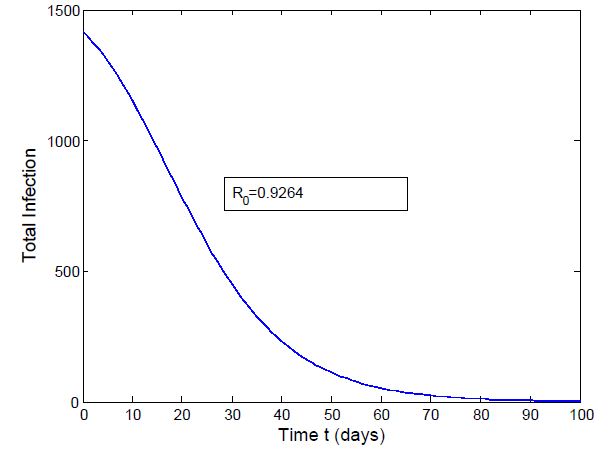 | Figure 3. Total Infection when  |
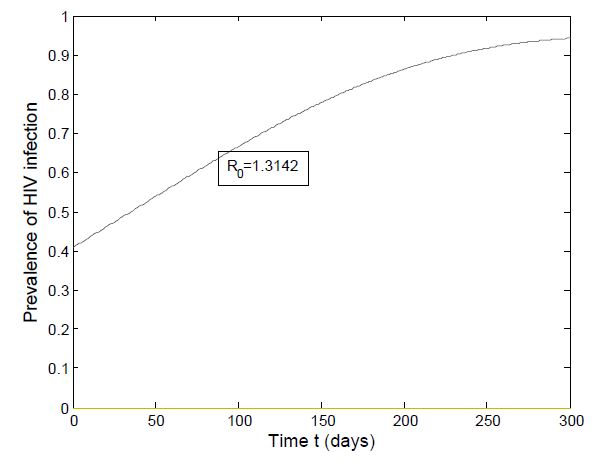 | Figure 4. Prevalence of HIV when 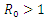 |
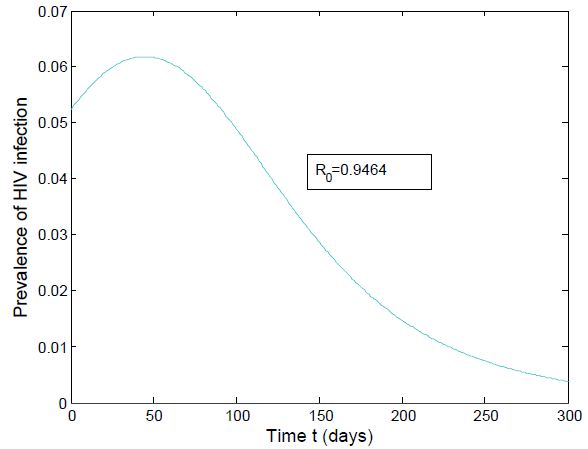 | Figure 5. Prevalence of HIV when 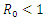 |
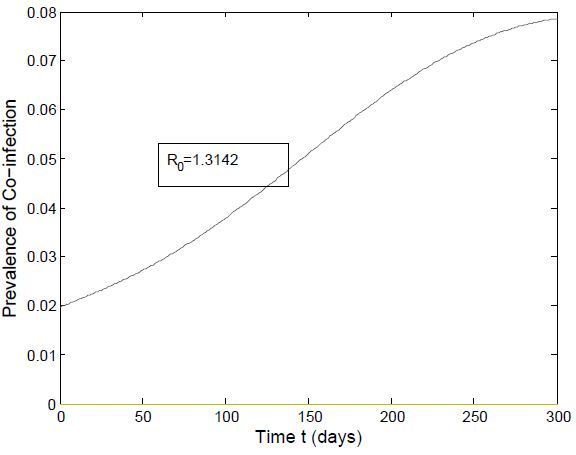 | Figure 6. Prevalence of Co-infection when 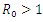 |
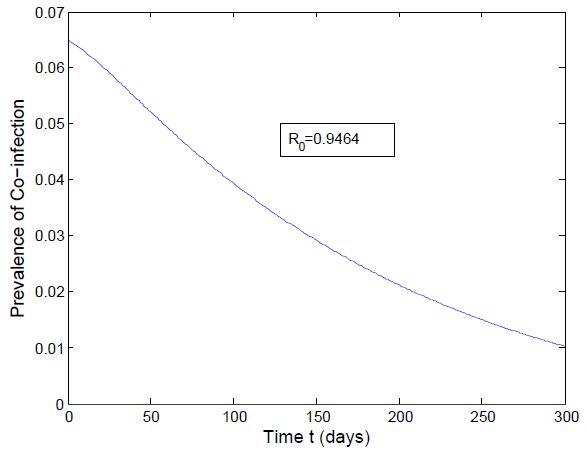 | Figure 7. Prevalence of Co-infection when 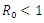 |
9. Conclusions
- HIV and malaria affect millions of people across overlapping geographic distributions. The risk of transmission of both diseases can be increased because of co-infection. Here we have formulated a deterministic mathematical model for the co-infection of HIV/AIDS and malaria in order to assess their synergic relationship. Positivity and boundness of solutions are analyzed quantitatively. Sensitivity indices of the basic reproductive number
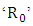 to the parameters in the model is calculated. Comprehensive mathematical techniques are used to analyze the model steady states. It is found that using the next generation matrices, the disease free equilibrium point is locally asymptotically stable when the reproduction number is less than unity and unstable when reproduction number is greater than unity. Centre manifold theory is used to show that the HIV/AIDS-malaria co-infection model`s endemic equilibrium are locally asymptotically stable when the associated reproduction numbers are less than unity. In summary, the main findings of this paper, if reduction in sexual activity of individuals with malaria symptoms decreases the number of new cases of HIV and the mixed HIV-malaria infection and also protecting HIV infective from mosquito bites.
to the parameters in the model is calculated. Comprehensive mathematical techniques are used to analyze the model steady states. It is found that using the next generation matrices, the disease free equilibrium point is locally asymptotically stable when the reproduction number is less than unity and unstable when reproduction number is greater than unity. Centre manifold theory is used to show that the HIV/AIDS-malaria co-infection model`s endemic equilibrium are locally asymptotically stable when the associated reproduction numbers are less than unity. In summary, the main findings of this paper, if reduction in sexual activity of individuals with malaria symptoms decreases the number of new cases of HIV and the mixed HIV-malaria infection and also protecting HIV infective from mosquito bites. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML