-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2024; 12(1): 8-19
doi:10.5923/j.als.20241201.02
Received: May 1, 2024; Accepted: Jul. 3, 2024; Published: Oct. 18, 2024

The Effect of DHA/EPA Rich Fish Oil on the Serum Markers of Myocardial Ischemia Induced in Rat
Deffo Tiepma Ngongang Flore1, Tiencheu Bernard1, Achidi Aduni Ufuan1, Mary Magdalene1, Zih Felicitas Enam1, Yolandia Jamea Nganje Epanty1, Agnes Namondo Mbongo Lyonga1, Tenyang Noel2
1Department of Biochemistry and Molecular Biology, Faculty of Science, University of Buea, Buea, Cameroon
2Department of Biological Sciences, Faculty of Science, University of Maroua, Maroua, Cameroon
Correspondence to: Deffo Tiepma Ngongang Flore, Department of Biochemistry and Molecular Biology, Faculty of Science, University of Buea, Buea, Cameroon.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cardiovascular diseases remain a major pathological cause of death worldwide despite rapid advancements made in the treatment of cardiac diseases. Dietary intake of omega-3 polyunsaturated fatty acid (PUFA) sources have proven to improve the prognosis of patients with symptomatic heart failure or myocardial infarction. This study therefore aimed at evaluating the effect of Decosahexaenoic acid/Ecosapentanenoic (DHA/EPA) rich fish oil extracted from cooked and raw mackerel fish (Scomber scombrus) on the serum markers of myocardial ischemia in isoproterenol-induced-rats. Raw and cooked mackerel fish oils were extracted using the Bligh and Dyer methods. The quality indices of the extracted oils were studied (Acid value, Saponification value, Peroxide value, Anisidine value and TOTOX value). A total of 42 adult Wistar albino rats were randomly and evenly distributed into 7 groups of six rats each, and then treated for 28 days. The normal group received distilled water (10ml/kgbw) orally, the positive control group received propranolol 10ml/kg plus Isoproterenol (ISO) 85mg/kg, the negative control group received only ISO, test groups one and two received cooked fish oil 8ml/kg and 10ml/kg respectively plus ISO and test groups three and four received raw fish oil 8ml/kg and 10ml/kg respectively plus ISO. After 28 days, the rats were anaesthesized, blood collected and centrifuged. Serum obtained was used for biochemical analyses (creatinine, lactate dehydrogenase, alanine amino transferase, aspartate amino transferase, alkaline phosphatase, gamma-glutamyl transferase, total cholesterol, high density lipoprotein, low density lipoprotein, very low density lipoprotein, triglyceride and albuminaemia). Results obtained after fat quality analysis of the oil showed that raw oil indices falls within the codex standard range and showed better quality as compared to cooked oil, as cooked fish oil free fatty acid, peroxide value, total oxidation and saponification values were significantly high (p≤0.05) as compared to that of raw fish oil showing that the cooked oil was more oxidized. Also the enzyme and lipid levels in serum were significantly lower (p≤0.05) in test groups as compared to the negative control showing that fish oil effectively prevented the disease. The present study showed that mackerel fish oil has cardio protective activity against isoproterenol-induced rats with raw fish oil showing greater effects as the fish oil significantly (p>0.05) decreased enzyme and lipid levels as well increased albumin levels in serum. Hence, supplement formulations of mackerel can be supported in replacement of available standard drugs that have cardio protective effects.
Keywords: Cholesterol, Lipoprotein, Fish oil, Myocardial ischemia, Scomber scombrus, Heart failure, Myocardial infarction, Omega-3, Polyunsaturated fatty acid, Cardiovascular diseases
Cite this paper: Deffo Tiepma Ngongang Flore, Tiencheu Bernard, Achidi Aduni Ufuan, Mary Magdalene, Zih Felicitas Enam, Yolandia Jamea Nganje Epanty, Agnes Namondo Mbongo Lyonga, Tenyang Noel, The Effect of DHA/EPA Rich Fish Oil on the Serum Markers of Myocardial Ischemia Induced in Rat, Advances in Life Sciences, Vol. 12 No. 1, 2024, pp. 8-19. doi: 10.5923/j.als.20241201.02.
Article Outline
1. Introduction
- Myocardial Ischemia is a pathological condition characterized by inadequate blood supply to the heart due to blockage or narrowing of the coronary arteries [1] Oxygen supply is thus restricted, ultimately resulting in myocardial cell death and hence infarction (heart attack). Increase risk and mortality of myocardial infarction due to ischemia, and other cardiovascular diseases can be attributed to arteriosclerosis, diabetes mellitus, untreated hypertension [2]. Cardiovascular disease (CVD) accounted for 32% of global deaths in 2019, making it the leading cause of deaths worldwide. More than 75% of the deaths caused by CVD occurred in low and middle-income countries, including sub-Saharan Africa [3]. In a survey carried out in Cameroon, it was proven that 33.6% of persons out of 100000 die of sudden cardiac arrest per year [4]. The elderly populations habour the highest prevalence of cardiovascular diseases [5] due to a strong positive correlation between increasing age and hypertension [6]. Evidences from animal studies, epidemiological studies, meta-analyses, and randomized controlled trials have proven that certain foods (dietary patterns) and individual dietary elements has an important role to play in the development and prevention of cardiovascular diseases. Changes in diet can lower blood pressure, prevent the development of myocardial ischemia, and reduce the risk of heart-related complications [7]. Some strategies for the prevention of cardiac arrest through diet include reducing sodium intake, limiting alcohol consumption, increasing potassium intake, and adopting an overall dietary pattern such as the DASH (Dietary Approaches to Stop Hypertension) or a polyunsaturated fatty acid rich diet(fish oils). In order to reduce the burden of blood pressure-related complications, efforts that focus on environmental and individual behavioral changes and that encourage and promote healthier food choices may be warranted [8]. Data from clinical [9] and experimental studies [10] support the hypothesis that consumption of n-3 PUFA lowers the risk of cardiovascular diseases and sudden cardiac arrest.Sea Fish oil is a very effective nutrient and contains important polyunsaturated n-3 fatty acids (n-3 PUFA) that can be absorbed easily [11]. Polyunsaturated fatty acids aids in reducing the risk of having cardiovascular diseases by modification of the cell membrane milieu on incorporation. Alteration of the lipid microenvironment in cardiomyocytes through the inclusion of omega-3 PUFAs can modulate ion function, leading to anti-arrhythmic effects. Also omega-3 PUFAs have shown anti-inflammatory effects by decreasing the circulatory concentrations of inflammatory cytokines and ameliorating left ventricular functional capacity [12]. Despite the consumption of these species rich in polyunsaturated fatty acids active molecules and active ingredients against cardiovascular diseases, the prevalence of myocardial ischemia, stroke, atherosclerosis and cardiovascular disease remains growing mainly due to oxidation of these molecules during cooking and/or culinary processes [13]. The increasing change in the eating habits of many Cameroonians where most people now consume fast foods and canned foods which are rich components of fat and salts leading to an increasing fats profile/osmolarity in the body hence leading to hypertension and therefore cardiovascular diseases, has led to the increase and prevalence of cardiovascular diseases in our society. Also, the nature of work of most Cameroonians has changed due to many people working in offices which do not lead to much energy use [14]. This can lead to accumulation of fats in the body and hence cardiovascular diseases. Drugs available in our pharmacist for cardiovascular diseases are very expensive and not easily affordable by the population. Many sources of PUFA (plant or animal origin) exist; among them are winter squash, brassica vegetables, wild rice, flax seed, hemp seed, blueberries, chia seed, canola, algae etc. are grown in Mediterranean and European zones and are rarely found in sub Saharan zones. In Cameroon people consume a lot of fish rich in polyunsaturated fatty acid that could help in the prevention of these cardiovascular diseases, but that is not the case because the fish is not eaten raw and during cooking the polyunsaturated fatty acid (PUFA) may have been destroyed. Extraction of virgin oil from raw fish rich in PUFA and producing them in capsule form as food supplement could be a promising solution to the increasing rate of cardiovascular diseases in our society as this will present a less expensive alternative to drugs. This work was aimed at extracting raw fish oil and determining its effect on serum markers of myocardial ischemia induced rats as a first step to the development of fish oil supplements, for the treatment of Ischemic heart diseases.
2. Materials and Methods
2.1. Sample Collection
- Atlantic Mackerel fish (scomber scombrus) was obtained from CONGELCAM-BUEA upon direct arrival of fresh stock.
2.2. Sample Preparation and Oil Extraction
- The fish obtained was cleaned and divided into two portions. Part of the fish was boiled for 30 minutes before grinding while the other part was ground raw with the aid of a hand mill. Both the raw and boiled fish were blended and oil extracted following the Bligh and Dye method with some slight modifications. For 100g of ground fish (both boiled and raw), 80ml of water was added followed 200ml of methanol and 400ml of chloroform. The mixture was then homogenized in a grinding machine (Panasonic, Kyoto, Japan), filtered and the aqueous layer (methanol-water) containing proteins, carbohydrates and phospholipids removed using a separating funnel. The chloroform (lipid layer) was evaporated using the rotary evaporator at 45°C. The oil obtained was aliquoted, then stored at ambient temperature until use.
2.3. Oil quality Assessment and Chemical Analyses of Fish Oil
2.3.1. Measurement of Free Fatty Acid
- The determination of free fatty acid (FFA) of fish oil samples was made according to the method described by AFNOR [15]. The results were expressed as % oleic acid (% FFA).
2.3.2. Analysis of Iodine Value
- The iodine value (IV) of fish oil samples was determined using the Wijs method, as described by AFNOR [15]. The IV was expressed as g I2 per 100 g of sample.
2.3.3. Peroxide Value
- The peroxide value was determined by referring to the IDF standard method, 74A: 1991 [16]. The results were expressed as milliequivalents of O2 per kg sample.
2.3.4. p-Anisidine Value
- Anisidine value was determined by the standard AOCS Cd 18–90 « p-anisidine value » using a Perkin Elmer UV‐Visible Spectrophotometer (Norwalk CT, USA) [17].
2.3.5. Total Oxidation Value
- Total oxidation (TOTOX) values of oil samples were determined using the equation TOTOX = 2PV + p- AnV according to [18].
2.4. Rat Bioassay
2.4.1. Ethical Clearance
- All procedures were carried out in accordance with the ethical clearance obtained from the University of Buea Institutional Animal Care and Use Committee with ref (2021/05/UB/IACUC/BTU/FS) of the 16/07/2021 date.
2.4.2. Animal Distribution and Grouping
- A total of 42 adults (three months aged) wistar albino rats, weighing 150 – 180g were purchased from the University of Dschang animal house and allowed to acclimatize to the laboratory conditions (temperature 24-27°C and 12hour light-dark cycle) in the animal house of the University of Buea for one week before commencement of the experiment. The rats were selected divided into 7 groups of 6; containing 3 males and 3 females each. The animals were given free access to solid pellet diet and water throughout the study. Table 1 summarizes the treatment administered to each group during the 28days of the study. The normal group received distilled water (10ml/kgbw) orally, the positive control group received propanolol as drug 10ml/kg plus Isoproterenol (ISO) 85mg/kg, the negative control group received only ISO, test groups one and two received cooked fish oil 8ml/kg and 10ml/kg respectively plus ISO and test groups three and four received raw fish oil 8ml/kg and 10ml/kg respectively plus ISO.
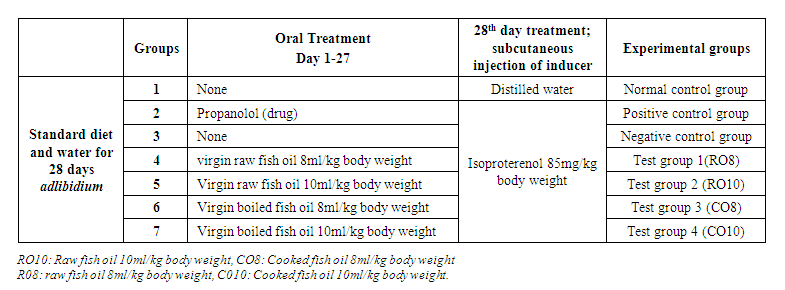 | Table 1. Treatment administered to the rats during 28days study |
2.4.3. Serum Analysis
- The following biomarkers were measured using standard commercial kits.
2.4.3.1. Albuminaemia and Serum Enzyme Activities
2.4.3.1.1. Albumin
- The basis of albumin concentration determination is the change of color of the complex formed between the protein and Bromocresol green (BCG) at acidic pH (4.3). The color change from yellow to green and then green to blue brings about a shift in absorption wavelength. The intensity of the formed is proportional t the concentration of albumin in the sample. The test procedure was as proposed by the kit manufacturer and included. The spectrophotometer was zero using distilled water. Into different cuvettes, 1ml of 0.12mmol/LBCG pH 4.3 was pipetted. In the first cuvette, 50ul of distilled water was added, in the second cuvette 50ul 5g/dL of albumin standard and in the third 50ul of serum sample. The cuvette with distilled water served as blank. The content in each cuvette was mixed and the absorbance read at 630nm. The albumin concentration in the sample was then determined using the formula of Rodkey [19].
2.4.3.1.2. Serum Enzymes
- Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were measured using the chrono lab enzymatic kit according to the methods describe by [20]. The concentration of creatinine was estimated using enzymatic kit ([21]. Alkaline phosphatase (ALP) was evaluted according to Njoku, Abarikwu [22] and Gamma- glutamyl transferase (Γ-GT) as describe by Pisano, Pacifico [23]. The activity of Lactate dehydrogenase was measured as absorbance during its catalytic oxidation of NADH to NAD+ according to the method describe by Feldman-Salit, Hering [24].
2.4.3.2. Estimation of Lipid Profile
- The triglyceride, Total Cholesterol level were determined by the Buccolo colorimetric method using Chrono lab test kit [25]. The very low density (VLDL) and low density (LDL) lipoproteins from serum or plasma were precipitated by phosphotungstate in the presence of magnesium ions. After centrifugation the supernatant contains high density lipoproteins (HDL) was determined using the total cholesterol enzymatic reagent [25]. LDL-cholesterol was calculated from measured values of total cholesterol, triglycerides and HDL- cholesterol according to the relationship: [LDL-chol] = [total chol] - [HDL-chol] - [TG]/5 and [TG]/5 was an estimate of VLDL-cholesterol [25].
2.5. Statistical Analysis
- Raw data was entered using Microsoft (MS) excel and descriptive statistics was carried out and values expressed as Mean ±Standard Deviation. The significance of difference amongst group was assessed using one-way analysis of variance (ANOVA), followed by t-test and statistical significance was considered at p-values less than 0.005.
3. Results
3.1. Mackerel Fish Oil Quality Indices
- The quality indices of the oils extracted from raw and boiled mackerel fish oil are represented in Table 2 below. Raw fish oil qualities were all within the WHO/FAO standard ranges whereas, boiled fish oil had values out of the standard range prescribed by Codex Alimentarius [26] except for the peroxide value.
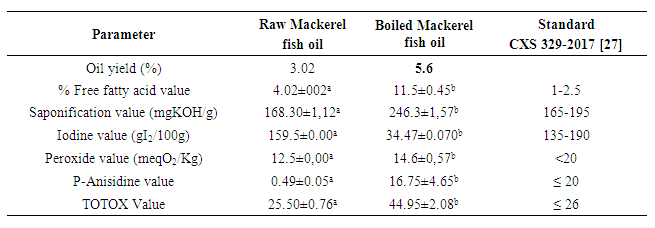 | Table 2. Quality indices of mackerel fish oil |
3.2. Animal Bioassay
3.2.1. Weekly Water Intake
- Figure 1 summarizes the weekly water intake of the rats for all the seven groups. For all the groups, during the last week prior to induction, the water intake decreased except for the normal group in which there was a general increase in water intake. However, the highest water intake was observed in the negative control group.
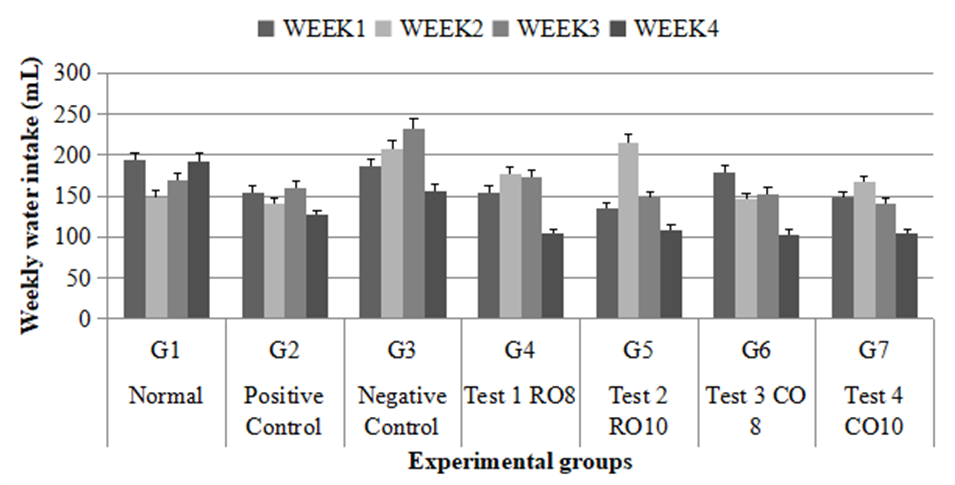 | Figure 1. Weekly water intake of experimental groups |
3.2.2. Weekly Food Intake
- There was a general decrease in food intake across all the groups during the fourth week prior to induction as shown on Figure 2 below. The food intake in the propanolol treated group (positive control) during the first week was highest.
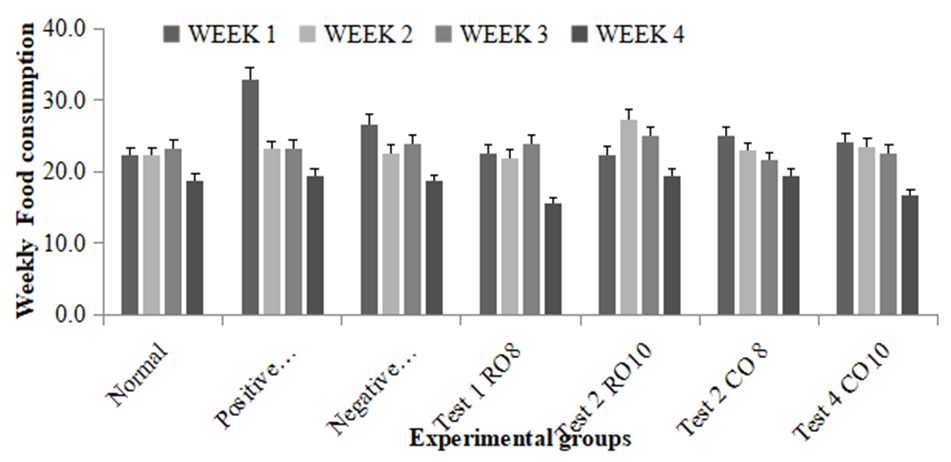 | Figure 2. Weekly food intake of the experimental groups |
3.2.3. Weekly Growth Response
- Growth response for across all the groups’ rats is summarized in Figure 3 below. The weekly growth response for the negative control group was lower than that of all the groups. However, across all the groups there was an increase in growth for the first till the fourth week except in boiled mackerel fish 8ml/Kg oil treated group in which there was a decrease in growth during the fourth week.
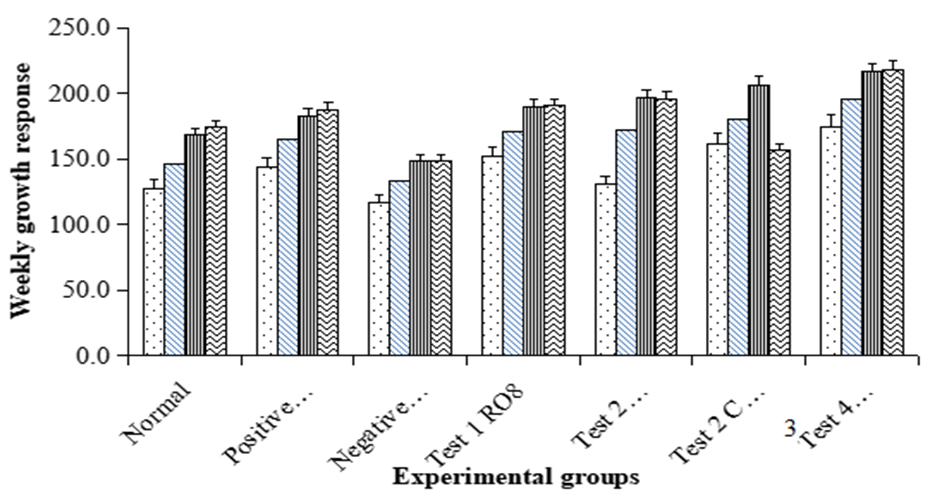 | Figure 3. Weekly growth response |
3.2.4. Effect of Mackerel Fish Oil on Serum Albumin and Enzyme Markers
3.2.4.1. Effect of Mackerel Fish Oil Treatment on Serum Albumin Concentration
- The total serum albumin level in the negative control (no-treatment isoprotenol induced myocardial ischemia) group was significantly lower than that of the normal control group, as shown in Figure 4. In rats pretreated with standard propanolon and the Mackerel fish oil treated rats, the reduced albumin levels were significantly increased, with raw mackerel fish oil treated having significantly higher albumin levels than the boiled Mackerel fish oil treated and propanolol treated groups.
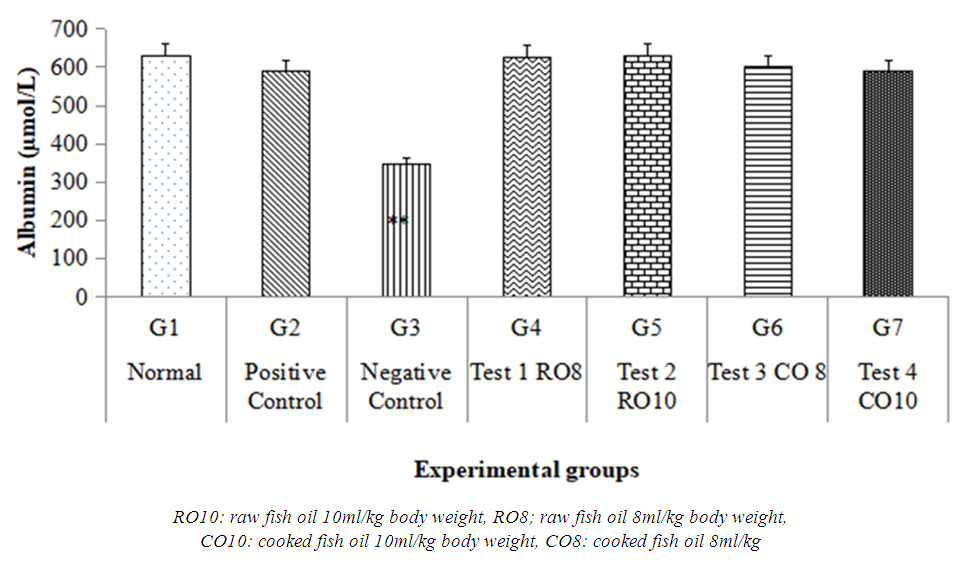 | Figure 4. Serum albumin concentration |
3.2.4.2. Effect of Mackerel Fish Oil Treatment on Serum Markers and Cardiac Markers
- Table 3 summarizes the serum marker concentrations and the concentration of cardiac marker in serum of the rats. Myocardial damage induced by isoprotenol caused significant increase in serum markers concentrations. The effect of treatment with Mackerel fish oil, showed significant reduction in the serum cardiac markers compared to the negative control group. Treatment with Mackerel fish oil showed significantly increased reduction in AST, ALT, GGT and LDH levels compared to the propanolol treated group (positive control). Similarly, the activities of the cardiac marker CK and LDH were reduced in the mackerel fish oil treated groups compared to the negative control group. Lower CK activity in fish oil treated groups was lesser than with positive control group however, greater than with the positive control.
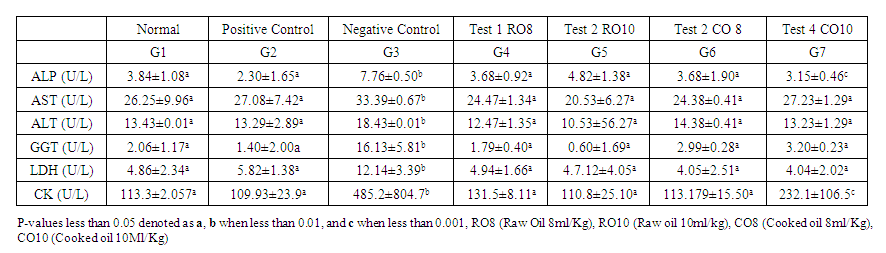 | Table 3. Serum markers concentration of the different groups |
3.2.5. Effects of Mackerel Fish Oil on Serum Lipid Profile
- The effect of Mackerel fish oil on serum lipids of the animals is summarized in Table 4 below. Generally, the administration of Mackerel fish oil both boiled and raw led to the improvement of the serum lipid parameters. The Raw Mackerel fish oil treated group showed significantly lower triglycerides, HDL, VLDL, LDL and Total cholesterol concentration compared to the positive control group.
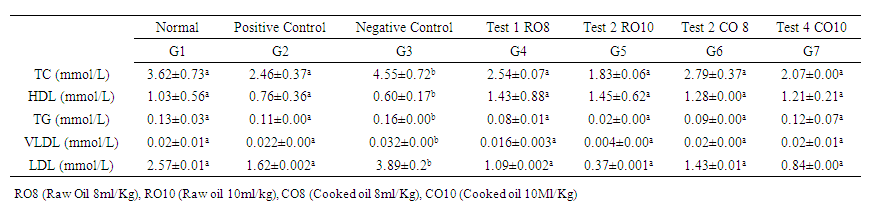 | Table 4. Serum concentration of lipid profile |
3.2.6. Effects of Mackerel Fish Oil on Organ Weights
- In this study generally, Isopreterenol-induced myocardial ischemia led to an increase in relative organ weights as shown in Figure 5 to 8.
3.2.6.1. Effect of Mackerel Fish Oil Treatment on Relative Heart Weights Per Group
- The relative heart weight (Figure 5) was significantly raised in the positive control groups than in the other groups. The raw Mackerel fish oil treated groups (RO8 and RO10) had relative heart weights significantly lower than the boiled Mackerel fish oil treated and negative control groups.
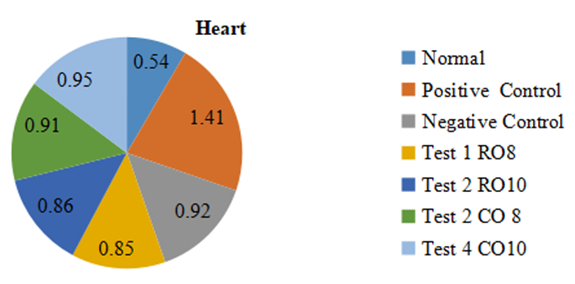 | Figure 5. Effect of Mackerel fish oil on Heart weights |
3.2.6.2. Effect of Mackerel Fish Oil Treatment on Relative Liver Weights of the Different Groups
- Figure 6 shows the relative liver weight of the rats per group. The relative liver weight observed in all the test groups and the negative control group was lower than that observed in the positive control group.
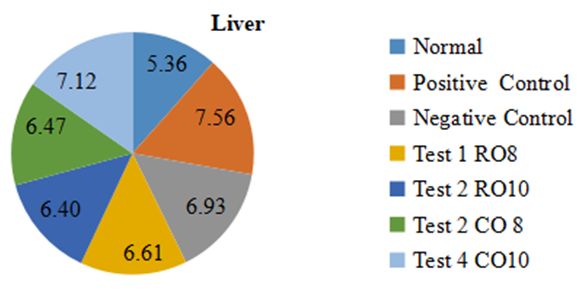 | Figure 6. Effect of mackerel fish oil on Liver weights |
3.2.6.3. Effect of Mackerel Fish Oil Treatment on Relative Kidney Weights Per Group
- The effect of treatment on the kidney weights is illustrated in Figure 7. The relative kidney weights of the raw mackerel fish oil treated groups are significantly lower than the positive control group for both the right and left (L) kidney. However, the boiled Mackerel fish oil treated groups showed relative right (R) kidney weights comparable to the normal control group and significantly lower than the other groups.
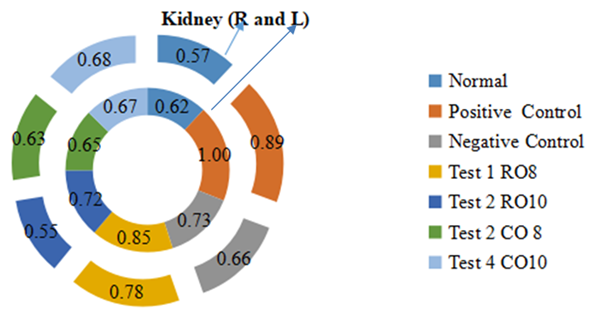 | Figure 7. Effect of Mackerel fish oil on Right and left kidney weights |
3.2.6.4. Effect of Mackerel Fish Oil Treatment on Relative Spleen Weights Per Group
- The relative spleen weights of all the groups is summarized on the pie chart (Figure 8). The positive control group showed a significantly elevated relative spleen weight compared to the other groups. The raw mackerel fish oil treated showed significantly lower spleen weights than the boiled Mackerel fish oil treated groups and the negative control group.
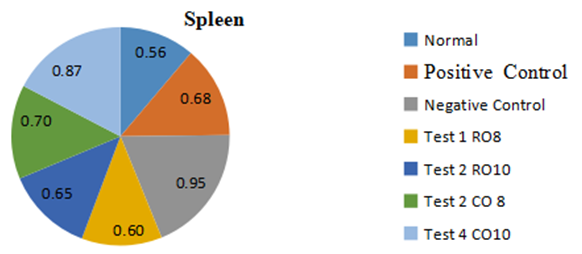 | Figure 8. Effect of Mackerel fish oil on Spleen weights of all groups |
4. Discussion
- Epidemiological studies indicate a low incidence of cardiac diseases in populations that consume large amounts of seafood. This effect is attributed to the presence of n-3 polyunsaturated fatty acids in these foods. In this study, the protective effect of fish oil against isoproterenol induced changes in cardiac markers, enzyme markers and lipid profile, were evaluated in albino Wistar rats. The rats were fed with raw and boiled Mackerel fish oil for 26 days prior to isoproterenol treatment on day 27 and day 28.The extraction yield of raw mackerel and boiled Mackerel fish oil using the Bligh and Dyer method were 3.02% and 5.6% respectively. Based on [28]. They can be classified as semi fatty fish (oil content 5-10%). Oil content less than 5% are lean fish while those with oil content above 10% are fatty fishes [28]. This classification of semi fatty fish is contrary to the normal classification of mackerel fish (sea fish) as reported by Cumin [29]. This can be due to the fact that the method of extraction used was (Bligh and Dyer) different from soxhlet used for the estimation of fat content. However, cooking improves the extraction yield of Mackerel fish oil by the Bligh and Dyer method.The higher acid value, saponification value, Iodine value, Peroxide value, P-anisidine value and TOTOX value of boiled Mackerel fish oil were higher compared to raw Mackerel fish oil can be attributed to the effect of heat (Womemi, 2009). However, raw Mackerel fish oil indices were within the standard values prescribed by CODEX Alimentarius Commission, Programme [30], for recommended international standards for fish oil, except for the acid value. According to Tenyang, Mawamba [31] acid value is a measure of the percentage of free fatty acids present in a fat. The higher the more prone to oxidation and hence shorter shelf life. The hydrolysis of triglycerides (Thermolysis) brought about by high temperatures during cooking may account for the significant variation in acid value for the boiled and raw Mackerel fish oils as reported Tiencheu, Womeni [32]. The value of 4.02% FFA obtained here was not too far from the recommended value for fish oil (≤2.5%FFA), therefore suggesting that it can be made fit for consumption by refining [33].Saponification value has been reported to be inversely related to the average molecular weight of the fatty acids in the oils, such that oils with saponification values less than 200 mg KOH/g have high molecular weight fatty acids [34]. Raw mackerel fish oil had 168mgKOH/g showing that the oil is rich in long chain fatty acids. The high saponification value of boiled mackerel fish oil 246 mgKOH/g of oil, on the other hand suggest destruction of the long chain fatty acids due to high temperature in boiling. The iodine value of boiled Mackerel fish oil revealed destruction of double bonds. It was (34.47gI2/100g) significantly lower than the value (108.09 gI2/100g) obtained by Adeniyi and Bawa [35]. This also suggests that Mackerel fish oil is particularly rich in unsaturated fatty acids as reported by Oladapo and Awojide [36]. Similar observation of iodine value was obtained by Tiencheu, Achidi [37] during processing of edible insects, R. phoenicis, and crickets.It has been shown that polyunsaturated fatty acids molecules are destroyed by high temperatures and even at room temperature due to the presence of a lot of pro-oxidant (oxygen, heat, metal) in fish oil [38].The peroxide value of both the raw and boiled Mackerel fish oils were below the threshold prescribed by the WHO/ FAO for edible oils (<20 milliequivalent of active oxygen/kg oil), however, greater than the threshold for fish oils prescribed by the CODEX (≤ 5 milliequivalent of active oxygen/kg oil) [39]. The peroxide value is a measure of primary oxidation product present in a fat sample. The higher Peroxide values in boiled than raw Mackerel fish oil suggest its deterioration caused by the alteration of unsaturated fatty acids, probably during boiling. Tenyang, Womeni [40] reported this same observation when studying effects of cooking and smoking on catfish (Arius maculatus).The secondary products of oxidation are measured from the anisidine value. The p anisidine values of both oils were below the threshold for fish oils prescribed by the CODEX (≤ 20). The oxidation rate of boiled fish oil is approximately 2 times higher than that of raw Mackerel fish oil. This is reflected by the TOTOX values. The total oxidation, TOTOX values of both oils were below the threshold prescribed by WHO/FAO standards for edible oils and CODEX standards for fish oils indicating that this oil were edible and less oxidized [39].An increase in the body weight of the rats was observed in all the groups from day 0 to day 28 can be attributed to the nutritive components received throughout the experiment There was no significant variation in in the food and water intake amongst all the groups, as such no significant variation was seen in the mean weight gain.Albumin measurements provide general information reflecting disease states in many organ systems. Albumin, as protein provides antioxidant scavenging capacities due to the presence of sulhydryl groups. Therefore, prevent lipids peroxidation and thus defense against oxidative stress [41]. Isoprotenol administration caused muscle damage and hence oxidative stress. The increase in albumin observed with the test oils can be attributed their involvement against oxidative stress. Albumin levels were brought to normal in groups pretreated with raw mackerel fish oil. The albumin levels in groups pretreated with cooked Mackerel fish oil and propanolol standard significantly increased even though not up to the normalization. These results are similar to that obtained by [41] in a study to assess the cardio protective effect of Rosa dementia in isoproterenol induced myocardial ischemia.Alkaline phosphatase is an enzyme present in all tissues that serve in the transport of substance across the cell membrane. ALP increase has been shown to be involved in the activation of inorganic phosphates and hence increase vascular calcification, oxidative stress and inflammation [41]. During injury ALP is secreted into the blood stream. ALP level was thus increased due to isoprotenol induction of myocardial ischemia. Treatment with Mackerel fish oil brought to normal the ALP levels where as the positive control propanolol cause reduction in ALPs levels to lower than the normal range. These results are comparable to that obtained by Chen, Shirakawa [42] who demonstrated that plasma Alkaline phosphatase activities were significantly lower in rat fed with fish oil indicating that inflammation in the rats was alleviated with PUFA. Aspartate amino transferase is a mitochondrial enzyme primarily found in the liver, heart and skeletal muscles in high concentrations. Isoproterenol induced oxidative stress causes mitochondrial injury that leads to cell necrosis, mitochondrial disintegration, and hence the release of AST into the blood stream [43]. Elevated levels of AST have been observed in myocardial injury. Treatment with Raw and cooked Mackerel fish oil led to a significant reduction in mean AST levels in all the groups compared to the positive control group. The lowest level was observed with 10ml/kg raw Mackerel fish oil fed group.Alanine amino transferase levels increases in the blood stream due to endothelial dysfunction induced by arteriosclerosis and inflammation [44]. ALT is present in high concentration in the liver and kidney and can be usually used in conjunction with AST in the diagnosis of myocardial infarction. ALT stays within normal range in the presence of elevated AST levels. Elevated levels of ALT observed in the isoproterenol treatment suggest significant membrane damage in the negative control group. Significant reduction in the levels of ALT was observed in Mackerel fish oil treated groups. The greatest reduction was seen with 10ml/kg raw mackerel fish oil treated group, suggesting greater protection against myocardial injury than propranolol standard and even cooked mackerel fish oil. These results are comparable to that obtained by Chen, Chen [45] who demonstrated that plasma ALT and AST activities were significantly lower in rat fed with fish oil indicating that inflammation in the rats was alleviated with PUFA. Gamma glutamyl transferase is an independent marker of cardiac death and cardiac reinfarction. It has been found to be involved in the pathogenesis of or arteriosclerotic and plaque stabilization [46]. Due to the administration of isoproterenol, γ-GT levels were significantly raised, however its levels were restored to normal in the standard propanolol pretreated group. The Mackerel fish oil pre-treated groups showed a greater reduction in γ-GT levels than the propanolol treated group. The 10ml/kg cooked mackerel fish oil treated group had the least effect while the 10ml/kg raw mackerel fish oil treated group showed the best effect. Similarly, the effect of 8ml/kg mackerel fish oil was greater with raw than with cooked mackerel fish oil.Lactate dehydrogenase enzyme is present in all body tissues but expressed extensively in blood cells and heart muscles. LDH can be measured as a surrogate marker of myocardial injury. Isoproterenol has been shown to cause increase in LDH levels [47]. Mackerel fish oil both raw and cooked showed cardioprotective effect by lowering LDH levels in isoproterenol induced myocardial ischemic rats. The effect was even greater than that of propanolol standard. There was no significant difference in the effect of Raw and cooked Mackerel fish oil.CK-MB in serum is an important marker in the diagnosis of myocardial injury. Oxidative stress may result in leakage of CK-MB into the general circulation [48]. In the present study there was a significant increase in the CK levels due to isoproterenol treatment as seen with the negative control group. However, treatment with mackerel fish oil caused a reduction close to normalization. 10ml/kg of raw mackerel fish oil produced a greater reduction compared to all the other groups whereas 10ml/kg of cooked mackerel treated group offered the least reduction. These results suggest that Mackerel fish oil helps in maintaining membrane integrity, thereby restricting CK-MB leakage.This study showed improvement in lipid profile of the isoprotenol induced myocardial ischemic rats. These results are in line with that reported by Al-Okbi, El-Qousy [49] with fish oil obtained from Iherb, 1795 World Wide Blvd, USA, and demonstrating cell protection by fish oil, Nigella oil, and Casined oil. Isoproterenol causes cellular cholesterol accumulation by increasing cholesterol synthesis, decreasing cholesterol ester hydrolysis and by reducing cholesterol efflux [50]. Pretreatment with mackerel fish oil restored the cholesterol levels. Raw mackerel fish oil caused a greater reduction in total cholesterol levels than cooked Mackerel fish oil. Increasing the concentration of the oil led to an increase reduction in the total cholesterol levels both in raw and cooked mackerel fish oil. 10ml/kg raw Mackerel fish oil fed group showed the greatest reduction, even more than the standard propanolol fed group.HDL-cholesterol is commonly referred to as “good cholesterol” because high levels are thought to reduce risk of cardiovascular diseases. HDL- cholesterol carries excess cholesterol from cells back to the liver. Favorable changes have been observed in HDL cholesterol due omega-3 consumption [51]. Mackerel fish oil treatment caused and increase in HDL-cholesterol levels in isoproterenol induced myocardial ischemic rats, even greater than the standard propanolol thus suggesting its potential rich n-3 PUFAs content. Raw Mackerel fish oil caused a greater increase than cooked Mackerel fish oil without regards to the concentration.LDL-cholesterol on the other hand is referred to as “bad cholesterol” it transports cholesterol, triglycerides, and other phospholipids into blood and play a key role in the formation of arteriosclerotic plaque. High levels of cholesterol bound to LDL is associated with myocardial infarction [52]. In this study, LDL level were significantly increased after isoproterenol treatment. Mackerel fish oil fed groups showed a significant reduction in LDL-cholesterol levels. The effect of Mackerel fish oil is concentration dependent; 10ml/kg treated groups showed lower levels of LDL compared to the standard propanolol treated and the other Mackerel fish oil treated groups. Raw mackerel fish oil conferred higher protection than cooked mackerel fish oil. Fish oil consumption has been shown to lower VLDL and triglycerides [53], similarly, in the present study the VLDL levels and triglycerides levels were significantly reduced in groups pretreated with mackerel fish oil. Very low density cholesterol like LDL is referred to as bad cholesterol this is because it transports and deposit cholesterol on the walls of the arteries. Thus the lowest its levels the better. Mackerel fish oil both raw and cooked caused a reduction in in the VLDL levels in the test groups greater than that due to propanolol standard. Cooked mackerel fish oil on the other hand brought to normalization the VLDL levels irrespective of the concentration. Raised triglycerides levels are primarily caused by increase in carbohydrates or saturated fatty acids. In this study, Mackerel fish oil fed groups showed a reduction in triglycerides levels even lower than the normal levels. This can be attributed to the PUFA rich nature of the oil. These results are in accordance with those obtained by Shang, Liu [54], that reported lower triglycerides levels both in serum and in the liver were found in animals treated with n-3 PUFAs. The results obtained in this study therefore show that Mackerel fish oil could be n-3 PUFA rich. Raw mackerel fish oil caused a greater reduction in serum triglycerides than in cooked Mackerel fish oil. This is probably due to the destruction of some of the PUFA by the high temperatures in cooking. Also, increasing the concentration of the raw Mackerel fish oil used caused a greater reduction in the triglycerides levels.The weight of internal organs can be used to establish whether or not the organ is pathological [55]. Investigating the effect of Mackerel fish oil on organs weights yielded favorable results as those of the serum cardiac markers and lipid profile in the present study. Elevated heart, liver kidneys and spleen relative weights were observed following isoproterenol administration. Elevated heart weight is evidence of myocardial hypertrophy, due to increased pulmonary resistance generally seen in obstructive coronary artery diseases can cause the right ventricle of the heart to enlarge in response to increased pressure [55]. Hepatomegaly is usually suggestive of liver disease, congestive heart failure or cancer [56]. Increase in spleen and kidney weights on the other hand have been associated with progression of acute heart failure to chronic heart failure [57]. Brain size has been shown to decrease with age and congenital heart disease [58]. The present study revealed that isoproterenol as well as propanolol standard and mackerel fish oils had no significant effect on brain weights. This is possibly due to the fact that Brain damage from isoproterenol induced myocardial ischemia had not been established yet. The short duration of the ischemia may not have been enough to cause brain damage. A second isoproterenol shot may be may be required in order to induce changes in the brain and hence observe the effect of Mackerel fish oil in the prevention of brain damage due to myocardial ischemia. The results obtained in the case of heart and liver weights are similar to those obtained by Al-Okbi, El-Qousy [49] with standard fish oil and giving more evidence to the effect of Mackerel fish oil in preventing myocardial ischemia.
5. Conclusions
- The oil quality indices in the present study suggested that the extracted oil were good edible fish oil. Though boiling increased the Mackerel fish oil yield, the high temperature in boiling also affected the oil quality indices, destroying the PUFAs present. Feeding the animals with raw or boiled Mackerel fish oil of different concentrations did not affect their growth or weight gain, but rather improved on the relative organ weights, serum and cardiac markers of myocardial ischemia, as well as the serum lipid profile of the animals, to different extend. Raw mackerel fish oil showed better oil qualities and hence significantly greater effect on cardiac markers, serum markers, and lipid markers of myocardial ischemia by decreasing triglycerides, low density lipoprotein, total cholesterol, and increasing high density lipoprotein and Albumin levels by protecting organs against toxicity. Also increasing the concentration of the raw fish oil significantly improved the levels of the markers in serum. Therefore, Mackerel fish oil is suitable be used in the prevention myocardial ischemia. Consumption of Mackerel fish will confer little or no protection against myocardial ischemia due to the post harvesting processing and high temperatures in cooking. This study suggests that mackerel fish oil can be formulated as supplements to prevent myocardial ischemia however, toxicity study, and improvement of extraction method to provide greater oil yield should be taken as a way forward. Refining the oil can also help in providing better oil quality indices to be used as supplement? Boiling should be considered preferred as a cooking technique as food security needs to be maintained since fish can’t be eaten raw. Mackerel fish (sea fish) should be most purchased as compared to other fish.
Conflicts of Interest
- The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
- Gratitude goes to the Agroecology Laboratory at the University of Buea for the realization of this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML