-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2024; 12(1): 1-7
doi:10.5923/j.als.20241201.01
Received: Jan. 26, 2024; Accepted: Feb. 22, 2024; Published: Mar. 4, 2024

Comparison Between the Effect of Carbohydrate Deprivation and Laparoscopic Sleeve Operation on Body Weight, Body Mass Index, pH, Insulin, and Leptin Levels
Ahmed F. Aldomairy1, Ashraf Kotb2, Ahmed Zaki3, Yasmine H. Eisa4, Radwa M. Elsabban5
1Assistant Professor of Anatomy and Embryology, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt
2Assistant Professor of Physiology, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt
3Assistant Professor of Surgery, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt
4Lecturer of Public Health and Preventive Medicine, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt
5Lecturer of Anatomy and Embryology, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt
Correspondence to: Yasmine H. Eisa, Lecturer of Public Health and Preventive Medicine, Faculty of Medicine, October 6 University 6th of October City, Giza, Egypt.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Obesity is a universal and an Egyptian issue and it is the cause of serious disorders. Carbohydrate deprivation diets and surgical bariatric procedures have become common obesity-fighting processes. In the present work, we compared between Karatay diet and laparoscopic sleeve operation outcome as regards body weight, body mass index, pH, insulin, and leptin levels after twelve weeks of the procedures. Sixty male patients with a body mass index of more than 40% were included in the study, According to their choice, half of them experienced carbohydrate deprivation (group I) while the other half underwent laparoscopic sleeve operation (group II). Written consent as well as institutional committee approval were done. The subjects showed a decrease in the following parameters; body weight, 20.2% in group I and 14.4 in group II, body mass index by 20.4% in group I and 14.5% in group II, pH level by 1.5% in group 1 and 0.13% in group II, fasting insulin level by 36.3% in group I and 3.1in group II, it showed also an increased leptin level by 136% in group I and 62% in group II. Both procedures were efficient in reducing body weight and body mass index. Carbohydrate deprivation showed higher results but with affection of pH, insulin, and leptin levels. Choosing one of these procedures should occur with caution.
Keywords: Carbohydrate, Karatay, Sleeve, Weight, Body mass Index, pH, Insulin, Leptin
Cite this paper: Ahmed F. Aldomairy, Ashraf Kotb, Ahmed Zaki, Yasmine H. Eisa, Radwa M. Elsabban, Comparison Between the Effect of Carbohydrate Deprivation and Laparoscopic Sleeve Operation on Body Weight, Body Mass Index, pH, Insulin, and Leptin Levels, Advances in Life Sciences, Vol. 12 No. 1, 2024, pp. 1-7. doi: 10.5923/j.als.20241201.01.
Article Outline
1. Introduction
- Carbohydrates are essential for a well-balanced diet and a healthy body, their most common source is grain foods such as bread, rice, and pasta. They are considered the only fuel source for many vital organs, such as the brain and kidneys. Carbohydrates are broken into simpler forms such as glucose, which is absorbed into the bloodstream, stimulating insulin secretion which is essential for cell entry. The excess glucose is stored as glycogen in the liver and muscles and in larger amounts as fat [1].Obesity is a worldwide issue. It is a main factor in developing serious diseases such as hypertension, diabetes, fatty liver, and other diseases [2]. In 2019, 5.020.000 (8.52%) of deaths in the world were attributed to obesity, and it was considered as the fifth cause of death. In the same year, Obesity in Egypt was attributed to 130.342 deaths as the second major cause of death [3]. In general, Egypt is the eighteenth country as regards obesity prevalence [4]. The prevalence of obesity has increased in adults in Egypt to reach about 40% according to The ‘100 million health’ survey (2019) compared to the 36% estimate of 2017 Stepwise survey [5].Carbohydrate deprivation diets such as Karatay and Atkins depend on fat and protein sources to generate energy and often recommend eating less than 100 gm of carbohydrates per day. Usually, carbohydrates are not completely restricted as they are needed in fat metabolism [6]. Typically, recommended food includes beef, chicken, bacon, fish, eggs, and non-starchy vegetables, as well as fats including oils, butter, and mayonnaise. Food that is restricted includes bread, serials, and other grains, most types of fruit, starchy vegetables, and dairy products other than cream or butter [7]. These types of diets are low in thiamine, folate, vitamins A, E, and B6, calcium, magnesium, iron and potassium, fiber, and lack important antioxidants and phytochemicals. They seem to be nutritionally inadequate if used for a long time [8]. The long-term safety is still uncertain, and the potential effects on a person’s health are not known. Follow-up studies are needed over years to determine that [9].The mechanism by which these diets act is the consumption of the glucose and glycogen stores in the liver and muscles by massive dietary carbohydrate restriction, as well as extra fluids exhaustion, around 3 gm of water is needed to release 1 gm of glycogen, so the rapid initial weight loss is mostly due to fluid loss [10]. Next, the body begins to depend on fat and proteins as a source of energy leading to the development of ketones in the body and acidosis [11].There is a controversy about the reflection of these results on the human body, carbohydrate deprivation diets result in convincing weight loss, lower blood glucose levels, and blood lipid profile improvement in the form of increased high-density lipoprotein and decreased triglyceride levels. although these changes are in favor of decreased risk of heart disease and prevention of diabetic complications [12], yet these diets are associated with a higher incidence of cardiovascular disorders and diabetes [13,14].The unusual food processing can lead to metabolic changes, which may be dangerous for some people, such as diabetic patients. Weight gain following cessation of carbohydrate deprivation diets is a common adverse effect, mainly due to body fluid restoration and muscle tissue rebuild, other effects include gastrointestinal disorders such as constipation due to restricted fiber intake, dehydration, nausea, loss of appetite, halitosis due to ketone bodies production and dizziness and lethargy due to unbalanced energy production [15]. Kidney problems and osteoporosis and related conditions due to loss of calcium [16]. On the other hand, laparoscopic sleeve gastrectomy has become an important modality in the treatment of obesity in the last decades, it involves the removal of approximately 75% of the stomach. Several mechanisms of weight loss were hypothesized including alterations in the secretion of hormones like ghrelin, peptide-YY, leptin, glucagon-like peptide, glucose-dependent insulin tropic peptide, decrease in insulin resistance, and alterations in gut microbiota together [17]. Laparoscopic sleeve gastrectomy has been considered a technically simple bariatric procedure with acceptable weight loss, resolution of comorbidities, and low postoperative complications, it is also easier to perform than other weight loss procedures such as laparoscopic gastric bypass. However, its complications can be more severe than those of other bariatric surgical techniques. The complication rates after laparoscopic sleeve gastrectomy vary among studies from 0% to 18%, with a 30-day postoperative mortality ranging from 0%-0.4% [18].LSG has a specific significant morbidity pattern including gastric staple-line leak [19], gastric fistula, obstruction or stricture [20], bleeding, and to lesser extent gastroesophageal reflux [21], nutrient deficiencies [22] and weight regain [23].
2. Objective
- This study was conducted to compare the outcome of marked carbohydrate deprivation and sleeve operation on body weight, BMI, pH, Insulin, and leptin levels.
3. Subjects and Methods
- Sixty patients complaining of obesity with a BMI above 35 were included in this study. Approval was obtained from the Institutional Ethics Committee. The candidates were visitors to nutritional clinics on October 6 and Cairo universities. All of them were on a free diet for the past 12 months, didn’t follow any diet regimen, and didn’t use any drugs affecting satiety or body weight. They were evaluated by careful clinical examination and laboratory investigation to exclude the presence of other abnormalities or chronic illnesses such as hypertension or diabetes. All the patients were suitable for treatment by either carbohydrate deprivation diet or sleeve operation, they freely selected the type of treatment they preferred. The patients were classified according to their BMI. WHO defines an adult with a BMI between 25 – <30 as overweight, and beyond 30 are considered obese [24]. Typically, both examined procedures are presented to subjects with a BMI equal to or beyond 35.To harmonize the two groups and for statistical purposes, only male patients were included, and we only included the first thirty patients of each group. According to criteria for bariatric surgery eligibility determined at the National Institutes of Health consensus development panel, patients had a BMI of 40 or more, BMI was calculated by dividing weight in kilograms by squared height in meters, failed to follow caloric diets and had no medical contraindications to surgery. A written consent was obtained from the patients. The participants were weighed, Insulin, leptin and pH levels were recorded at the beginning and the end of the study. Accordingly, the following groups were included in the study:Group I (Carbohydrate deprivation group): Consisted of thirty patients and they followed a carbohydrate deprivation diet for twelve weeks. These subjects were directed to consume their regular daily content of food and fluid but with maximal carbohydrate deprivation. The diet used in this study was Karatay diet. Tissues that require glucose can have their energy needs filled by the small amounts of carbohydrates from the diet or from gluconeogenesis, in which glucose is manufactured from other substances [25]. Group II (Sleeve group): consisted of thirty patients and they had a sleeve operation and followed for the following twelve weeks. Statistical analysis: The data were encoded and analyzed using the Statistical Package for the Social Science computer software (SPSS, version 20.0, SPSS Inc., Chicago, IL, USA).
4. Results
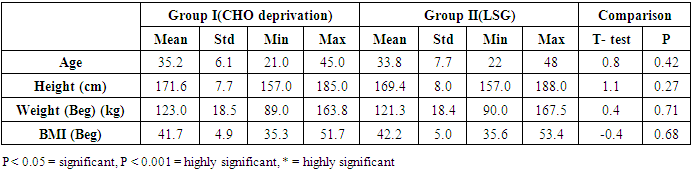
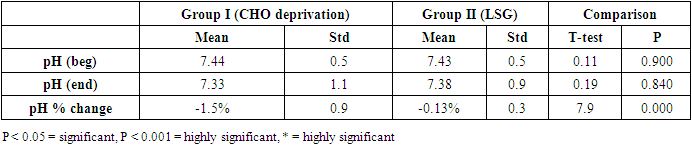
- - The data obtained at the beginning of the study showed no statistical difference between the two examined groups as regards age, height, weight, and BMI Table (1).
|
|
|
|
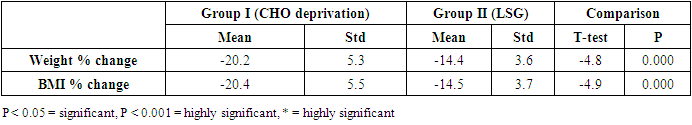
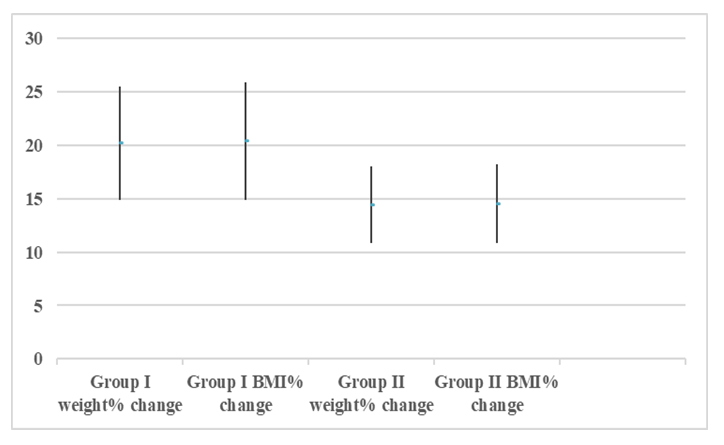 | Figure (1). Showing the mean, ±STD of the weight% change and BMI% change of the two groups |
|
|
|
5. Discussion
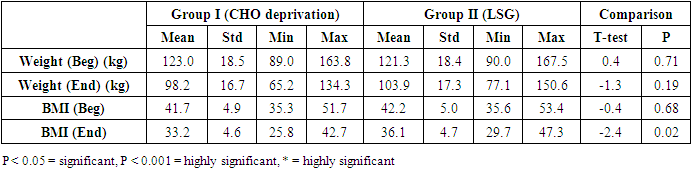
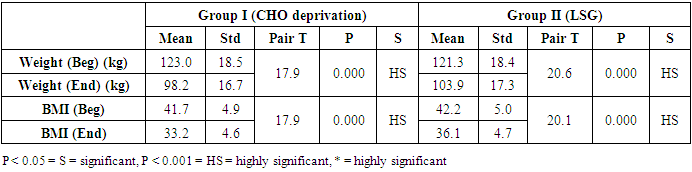
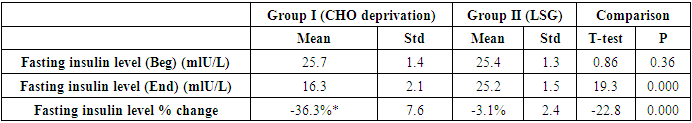
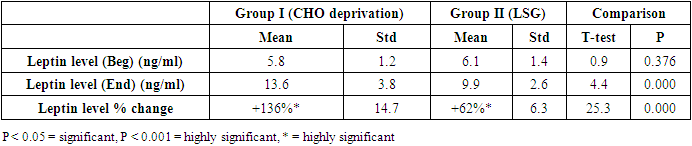
- The results of the present study showed a significant decrease in body weight and BMI by more than 20% at the end of the trial in the carbohydrate deprivation group compared to the initial measures at the beginning. The mechanism of the rapid obvious change is previously reported, when carbohydrate intake decreases to less than 100 mg/day, carbohydrate stores are significantly decreased and the body metabolism changes. With the presence of fatty acids, activation of the ketogenic pathway occurs, and the body, rather than relying on glucose, will use alternate substances in energy production, these include fatty acids, which are harvested from fat cells, and ketones derived from proteins as well as fluid loss [26]. A study recently declared that the changes in the body metabolism after changes in body weight either by dietary calorie restriction or by bariatric surgery are the same with minimal differences that are not significant in the long-term management of obesity. In the present work, the carbohydrate deprivation group showed a highly significant decrease in the rate of change in weight and BMI compared to the sleeve operation group, indicating the higher efficacy of this type of regimen in decreasing body weight and BMI than undergoing gastric sleeve operation. [27]. A significant decrease in the pH of the blood is noticed in the carbohydrate deprivation group which showed a 1.5% decrease at the end of the trial compared to the initial values detected before the beginning of the study. The regular decrease in pH values indicates that changes in the blood pH may increase over time. Accordingly, subjects following this type of regimen should not continue it for a long time and should hold on in between the trials to prevent marked affection of the pH of blood or acidosis. In clearer text, a Carbohydrate deprivation regimen should not be considered a style of life or lifelong regimen. That is why [28] recommended that the duration of the ketogenic diet should range from a minimum of 2–3 weeks to a maximum of 6–12 months to avoid a metabolic condition named “physiological ketosis”. The results of the study also showed a nonsignificant decrease in the pH of the blood by 0.13% in the sleeve group compared to the initial value before the operation. This indicates that this operation hazards through the pH change are negligible.By the end of the study, a highly significant decrease in insulin level by about 36.3% in the carbohydrate deprivation group was detected. This is attributed to the fact that carbohydrates are the main stimulus of insulin release [29]. The sleeve group showed a decrease in insulin level by 3.1% which may be attributed to the stress accompanying the operation. Comparing the two examined groups, a highly significant difference was found as regards the insulin change rate, this indicates the effectiveness of carbohydrate deprivation in decreasing insulin levels and hence the weight reduction. Again, lack of control of insulin level variation is another concern in carbohydrate deprivation regimen. The results of the study showed a highly significant increase in leptin levels in the two examined groups by 136% in the carbohydrate deprivation group and 62% in the sleeve group. The comparison between the two groups showed a highly significant difference. Leptin is secreted mainly by white adipose tissue, and levels are positively correlated with the amount of body fat [30]. It is secreted in a pulsatile fashion and has a significant diurnal variation [31]. Circulating leptin levels reflect the amount of energy stored in fat and acute changes in caloric intake [32].Leptin circulates in blood and acts on the brain to regulate substance intake and energy expenditure, it also suppresses appetite until weight is lost. Leptin mediates its effects by binding to specific leptin receptors (ObRs) expressed in the brain as well as in peripheral tissues. The ObRa isoform plays an important role in transporting leptin across the blood-brain barrier [33]. The ObRb isoform mediates signal transduction and is strongly expressed in the hypothalamus, an important site for the regulation of energy homeostasis and neuroendocrine function [34]. The binding of leptin to the ObRb receptor activates several signal transduction pathways which are important for the regulation of energy homeostasis, which is important for regulation of both food intake and glucose homeostasis [35].leptin activates a complex neural circuit comprising of anorexigenic and orexigenic neuropeptides to control food intake. Outside of the hypothalamus, leptin interacts with the mesolimbic dopamine regulation, which is involved in motivation for and reward of feeding, and the nucleus of the solitary tract of the brainstem contributes to satiety [36]. Other effects of leptin involving regulation of immune function, and bone metabolism are under intense investigations but are beyond the scope of this study. Accordingly, the prominent leptin level changes found in this study, although accompany, cause, and support the body loss condition, yet should be treated with caution. A study [37] mentioned that caloric restriction changes in leptin activity reflect adaptations to weight loss, but they have no impact on weight or fat regain. The results of this study point to the needed close follow-up in the two examined procedures, especially the carbohydrate deprivation in which the changes were more striking as regards pH, insulin, and leptin levels. Further studies are needed to explore the long-term side effects of the two examined procedures with more precautions regarding carbohydrate deprivation regimen.
6. Conclusions
- From the results of the present study, it is concluded that marked carbohydrate deprivation is more effective in body weight and BMI reduction than sleeve operation. However, the accompanying noticeable effect on blood pH as well as insulin and leptin levels should limit its use to short, interrupted periods. In general, choosing one of both examined procedures should be done after complete clarification of their possible side effects and calculating the benefit-issue of each of them.
7. Declarations and Conflict of Interest
- Author contributionsAshraf Kotb and Ahmed Zaki contributed to the study conception and design. All authors shared the development, material preparation, and data collection Ahmed AlDomairy and Radwa Elsabban performed and processed the physical measurements of the patients as well as the anatomical evaluation of the radiological studies. The principle of the article and the first draft of the manuscript was written by Ahmed AlDomairy, Yasmine Hamdy managed the processing and interpretation of the statistics. All authors performed the literature search and revised and commented on previous versions of the manuscript. All authors read and approved the final manuscript. Funding and Competing interestsThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.Compliance with Ethical StandardsApproval of the study was obtained from the Institutional Ethics Committee of October 6 University (approval number PRC-Me-2104012). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Data availabilityAll data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML