-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2021; 10(1): 14-20
doi:10.5923/j.als.20211001.02
Received: Jan. 15, 2021; Accepted: Jan. 30, 2021; Published: Feb. 6, 2021

Comparative and Morphological Appearance of Blood Cell in Some Ducks and Fowls
Thida1, Khin Cho Myint1, Ni Ni Soe1, Hsu Htoo2, Kyi Kyi Mar1, Wut Yee Nandar1
1Department of Zoology, East Yangon University, Yangon, Myanmar
2Transgenic Research Center, School of Life Sciences, Northeast Normal University, Changchun, China
Correspondence to: Khin Cho Myint, Department of Zoology, East Yangon University, Yangon, Myanmar.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The blood samples were collected from the 20 numbers for 10 samples of fowls namely Gallus domesticus and Gallus gallus domesticus and 10 samples of ducks namely Anas platyrhynchos and Anser cygnoides. The blood samples of ducks were collected from Thongwa environs and the samples of fowls were collected from Theinphyu market. Yangon Region. The study period was from June 2015 to Dec 2016. Stained blood smears using wedge smear technique and Leishman stain was prepared. Blood cells were examined by X 1000. Cells types were identified based on well shape and mean size, nucleus shape and mean size in diameter and staining properties. The morphological and cytochemical features of erythrocytes and leucocytes thrombocytes of studied species were compared and discussed.
Keywords: Blood cell shape and size, Nucleus shape and mean size in diameter
Cite this paper: Thida, Khin Cho Myint, Ni Ni Soe, Hsu Htoo, Kyi Kyi Mar, Wut Yee Nandar, Comparative and Morphological Appearance of Blood Cell in Some Ducks and Fowls, Advances in Life Sciences, Vol. 10 No. 1, 2021, pp. 14-20. doi: 10.5923/j.als.20211001.02.
Article Outline
1. Introduction
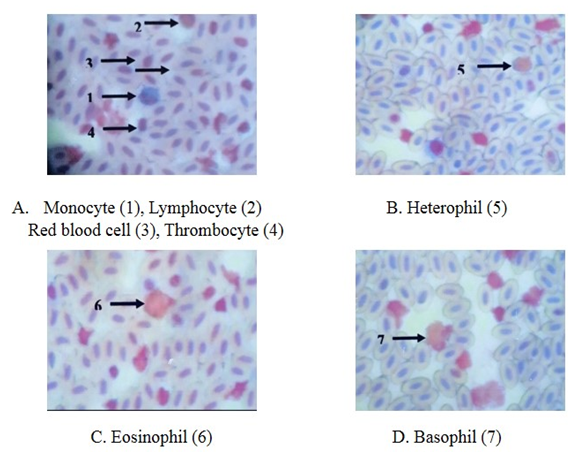
- In all vertebrates the blood comprise (1) a nearly colorless plasma, (2) white blood cell or leucocytes of several kinds, (3) red cells or erythrocytes. Colored by the contained hemoglobin which serve to transport oxygen and (4) smaller cells, the platelets, or thrombocytes. The plasma carries dissolved foods, wastes, internal secretions, and some gases (Storer and Usinger, 1979). Blood is a specialized body fluid in animals that delivers necessary substances such as nutrient and oxygen to the cells and transports metabolic waste products away from those same cells (Albers and Sanderson, 1996). Haemotological and blood chemistry values can be measured easily and are useful in determining the health on several condition of birds (Siegel, 1968).The blood contains a fluid plasma in which blood cells called corpuscles are suspended unattached to one another. Plasma is about 55 percent and corpuscles are 45 percent (Jordon, 2004). Red blood corpuscles (erythrocytes) contain iron, water, various inorganic salts and haemoglobin; they carry oxygen and help in removing carbon dioxide. There are two main types of white blood corpuscles or leucocytes, according to the shape of nuclei and presence or absence of granules in their cytoplasm. They are granulocytes and non-granular leucocytes. Granulocytes or granular leucocytes have numerous granules in cytoplasm and the nucleus is segmented into two or more lobes, they are of three types, basophils, eosinophils and neutrophils. Non-granular leucocytes may or may not have small granular of neutral nature but the nucleus is never segmented into lobes. These leucocytes are lymphocytes and monocytes (Jordon, 2004).In vertebrates, except mammals, the blood has thrombocytes or spindle cells, they are small in size with clear cytoplasm and an oval or rounded nucleus, and they help in clothing of blood (Jordon, 2004). Blood cell types and their morphology form an important part of disease diagnosis in groups of animals. By analyzing blood cell characteristics, diseases status can be identified (Anderson, 2008). In the warm-blooded birds and mammals the blood by differentials distribution between the internal organs and the body surface, serves to maintain the temperature of the entire body within close limit. Finally, the blood is the defense mechanism against foreign organisms having therefore a major role in maintain normal health and opposing the effects of infection (Store and Usinger, 1979).The consumption of healthy and cleaning meats including fowls and ducks is important for health all over the world. Nowadays, the poultry farms occurred some infection of virus such as avian influenza. Thus, the good and healthy poultry farms may be breed in Myanmar. Expecting to get the normal hematological value and parameter from the studied species.
2. Objectives
- This study was conducted to determine and compare the morphology of blood cells in some fowls and ducks. Therefore, this study is based on the following goals.1. to examine composition of blood cell types and their morphology in the studied species2. to determine and measure the mean diameter of the blood cells and their nuclei in the studied species3. to compare the obtained data between the studied species
3. Materials and Methods
3.1. Study Area and Study Period
- The ten blood samples from the two species of birds (duck), namely Anas platyrhynchos (Linnaeus, 1758) (Mallard) and Anser cygnoides (Linnaeus, 1758) (Goose) were collected from the Thongwa Township and other ten samples of the two species of birds (fowl), namely Gallus domesticus (Linneaus, 1758) and Gallus gallus domesticus (Linneaus, 1758) were collected from Theinphyu Market. Yangon Region.
3.2. Study Period
- The study period lasted from June, 2015 to December, 2016.
3.3. Blood Sample Collection and Preparation
- The right wing was extended fully and the medial aspect of the humeral area was sterilized with a cotton wool soaked in methylated spirit. Blood samples were collected from the brachial vein of the extended wing. The blood was drawn smoothly into a three ml syringe with an 18 gauge needle. The blood was collected in vacuum tube containing Ethyiene Diamine Tetra Acticacid (EDTA). Blood sample of 1 ml each was mixed into 3 ml of EDTA in a vacuum tube. The blood was mixed with the anticoagulant as soon as possible after collection by gentle rotation or inversion. EDTA treated samples are preferred for general haematological work. Basic experiments on haematology were done using routine thin blood film preparation and staining. The method was after Dacie and Lewis (1975) (Plate 3). The haemotological analysis was done at laboratory of Department of Zoology, East Yangon University.
3.4. Thin Blood Film Preparation
- Freshly drawn blood sample must be used in preparation of a thin blood film. Clean, grease-free slides with smooth unbroken ends were chosen. Place the slide on the counter or other horizontal surface. Place a medium sized drop of blood about 24 mm from the right end, midway between the edges of the slide. Hold this slide by pressing downward on the left hand. Take another slide, the spreader, in the right hand, holding it by its right end and place it in the center of the flat slide so that it forms an acute angle with the surface of the horizontal slide. Draw the spreader to the right until its end comes in contact with the drop of blood. Stop at this point and allow the blood to spread along the acute angle by capillary action. Before the drop has spread to the edges of the horizontal slide, push spreader to the left in one continuous sweep. This pulls the thin blood film along. Smears should be thin and uniform, thickness is governed by (1) the size of the drop and (2) the angle of the spreader. An acute angle produces a thin smear. When the film is spread hasten drying with a little heat or current of air. Rapid drying of the film prevents crenation and fragmentation of the erythrocytes. Stain immediately and store in dust-free box to prevent flies, cockroaches, etc, from eating holes in the blood smear (Lewis, 1976).
3.5. Staining of Thin Blood Film
- Leishman stain was carefully dropped onto the thin blood film so as to cover the whole thin blood film. The stain on the thin blood film was left for about 2 minutes. Then distilled water was added to the stain on the thin blood film to dilute. When a treat pipette, the diluted stain is then sucked up and down to mix it thoroughly. A correct dilution of the fluid will have a thin greenish scum. The diluted stain was allowed to act for 10 minutes after which it was washed off with distilled water, stand the slides for two stain an end to dry.When dry, a liberal drop of immersion oil in the surface of the smear and proceed with the examination by oil immersion. Examined a large portion of the smear progressing from one edge to the middle and back again be sure to include the edges of the smear during this meandering. Continue this pattern until a representative area has been covered. The method was after Lewis (2000).
3.6. Formula of Leishman’s Stain
- Leishman’s stainingLeishman’s powder ------------------------- 0.2 gmMethanol ------------------------------- 100mlDirections0.2 gm of powdered dye is weighted out and transferred to a conical flask of 200-250 ml capacity. 100ml of methanol are added and the mixture is warmed to 50°C for 15 minutes with occasional shaking. The solution is filtered. It is them ready for use, but will improve on staining (Lewis, 2000).
3.7. Measuring the Blood Cells
- Mature erythrocytes, leucocytes and thrombocytes and their nuclei were measured for diameter in each species of bird: and their mean diameter values in microns were calculated in each species followed after Murry (1992).Formula for calculation of standard deviation
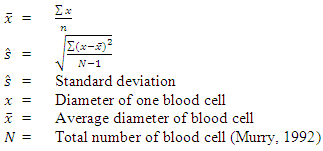
3.8. Identification of Birds and Blood Cells
- Morphological identification of erythrocytes, leucocytes, and thrombocytes were followed after Schermer (1958) and Thein (1979). The classification of the studied birds was followed after Robson (2005).
4. Results
- The characteristic of the morphology of the erythrocypes, leucocytes and platelets (thrombocytes) in ducks and fowls were described.
4.1. Systematic Position of the Studies Duck Species
4.1.1. Blood Corpuscle of Anas platyrhynchos
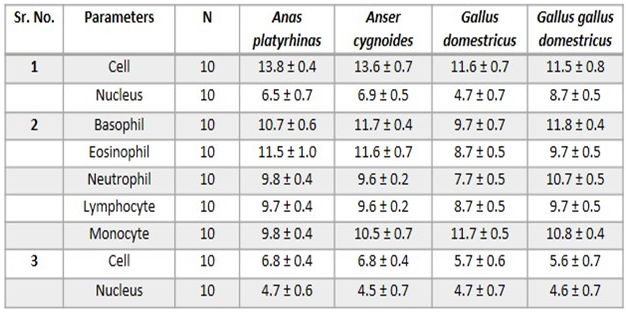
- Erythrocytes (RBC)The red blood cells were flat oval disc with a biconvex nucleus which was oval in shape center. The mean diameter of the erythrocytes was 13.8 ± 0.4 µm and the mean diameter of nucleus was 6.5 ± 0.7µm (Plate 1 and Table 1).
 | Plate 1. The blood cells of Anas platyrhynchos, Leishman stain (X 1000) |
4.1.2. Blood Corpuscle of Anser cygnoides
- Erythrocytes (RBC) The red blood cells were flat oval disc with a biconvex nucleus which was oval in shape in the center. The mean diameter of the erythrocytes was 13.6 ± 0.7µm and the mean diameter of nucleus was 6.9 ± 0.5µm (Plate 2 and Table 1).Leucocytes (WBC)GranulocytesThe basophil had large granules and nucleus was twisted with three lobes constrictions. The mean diameter of basophil was 11.7 ± 0.4 µm. The eosinophil had large granules and the nucleus with two lobes connected by a thread. The mean diameter of eosinophil was 11.6 ± 0.7 µm. The neutrophil had fine granules and the nucleus with only lobe. The mean diameter of neutrophil was 9.6 ± 0.2 µm (Plate 2 and Table 1).Non-granular leucocytesThe lymphocytes were round and had a nucleus with an indentation. The mean diameter of lymphocyte was 9.6 ± 0.2 µm. The monocyte was kidney shaped. It had more cytoplasm than lymphocytes. The mean diameter of monocytes was 10.5 ± 0.7 µm (Plate 2 and Table 1).
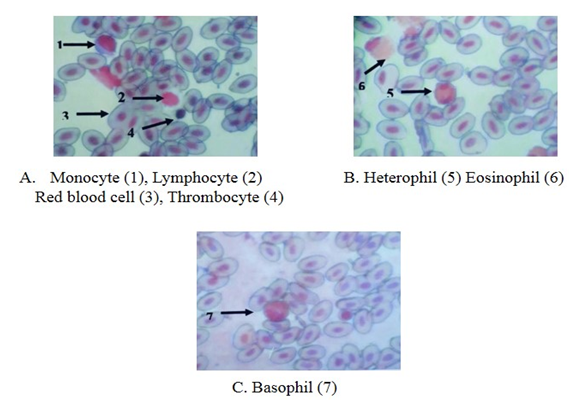 | Plate 2. The blood cells of Anser cygnoides, Leishman stain (X 1000) |
4.2. Systematic Position of the Studied Fowl Species

4.2.1. Blood Corpuscles of Gallus domesticus
- Erythrocytes (RBC)The red blood cells were flat oval disc with a biconvex nucleus which was also oval in shape found to be in the center of the blood. The mean diameter of erythrocyte was 11.6 µm and the mean diameter of the nucleus was 4.7 µm (Plate 3 and Table 1).
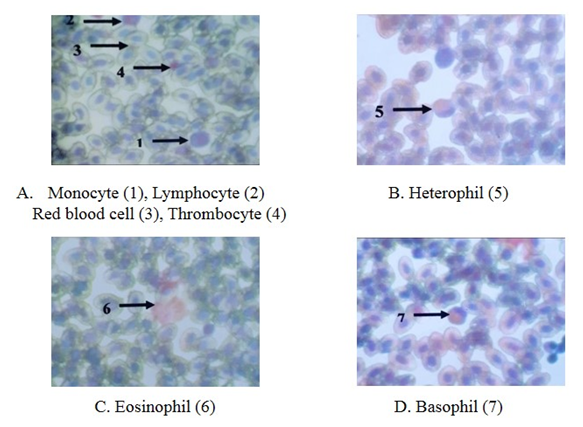 | Plate 3. The blood cells of Gallus domesticus, Leishman stain (X 1000) |
4.2.2. Blood Corpuscles of Gallus gallus domesticus
- Erythrocytes (RBC)The red blood cells were flat oval disc with a biconvex nucleus which was also oval in shape found to be in the center of the blood. The mean diameter of erythrocyte was 11.5 µm and mean diameter of the nucleus was 8.7 µm (Plate 4 and Table 1).
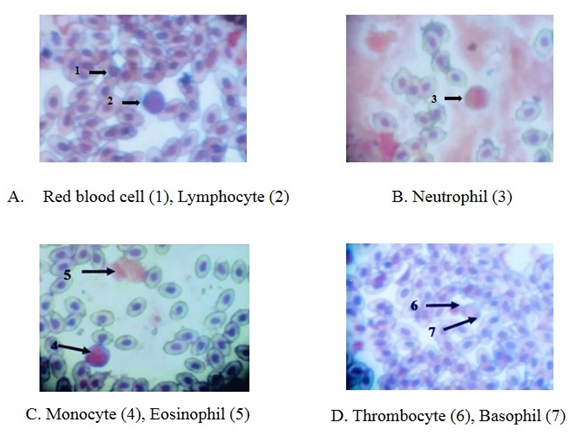 | Plate 4. The blood cells of Gallus gallus domesticus, Leishman stain (X 1000) |
|
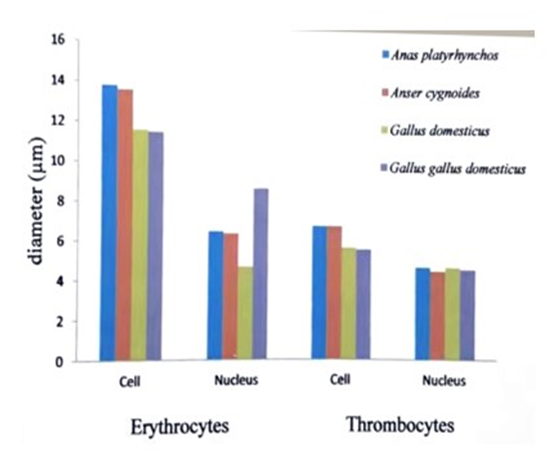 | Figure 1. Comparison of mean diameter of red blood cells and thrombocytes (platelets) and their nuclei in the studied bird species |
 | Figure 2. Comparison of mean diameter of white blood cells of their studied bird species |
5. Discussions
- In all vertebrates the blood comprises (1) nearly colorless plasma (2) white blood cells or leucocytes (3) red blood cells or erythrocytes (4) platelets or thrombocytes (Tibbo, 2004).In this study, the blood sample of three species and one subspecies, Anser cygnoides,Anas platyrhynchos, Gallus domesticus and Gallus gallus domesticus were collected. From these samples, the characteristics of the morphology of the erythrocytes, leucocytes and platelets were described.The red blood cells of the birds are oval and biconvex. The nuclei are also oval shape. In Corvus splendens splendens, Lonchura ferruginosa atricapilla, Lonchura punctulata lineoventer, Streptopelia chinensis tigrina, Passer domesticus confucius, Porphyrio poliicephalus, the largest red blood cells were observed in Convus splendens splendens (18µm) and the smallest cells were found Passer domesticus confucius (6 µm) (Thein H., 1979).The five studies of birds, A.platyrhynchos, C. japonica, G.domesticus, G.gallus and G. g domesticus have been domesticated in Myanmar for a long time. The mean diameter (nearly 12 µm) and nucleus of red blood cell and was equal (nearly 6 µm) in studied birds species (Khin, et.al., 2014).The mean diameter of red blood cell of Conturnix japonica (13 µm) was larger than the red blood cells of Gallus gallus (11 µm). The mean diameter of nucleus of red blood cells of C.japonica (7 µm) was also larger than G.gallus (6 µm) (Term paper, Group XXX VI 2013).In this study, the largest mean diameter of red blood cells was found in Anas platyrhynchos (13.8 µm) and smallest in Gallus gallus domesticus (11.5 µm). And the largest mean of the diameter of nucleus of red blood cell was found in Gallus gallus domesticus (9 µm) and the smallest in Gallus domesticus (5 µm).Among the birds, the largest basophiles were observed in Porphyrio porphyrio policephalus (12 µm) and the smallest one were observed in Lonchura punctulata lincoventer (11 µm) (Thein H., 1979).The mean diameter of basophils (11 µm), eosinophils (10 µm) and neutrophils (10 µm) were nearly equal size in studied species (Khin et.al., 2014).The mean diameter of basophil of Coturnix japonica (12 µm) was larger than the basophil of G.gallus (11 µm) (Term paper, Group XXX VI 2013).In this study, the greatest mean diameter of basophils was observed in Gallus gallus domesticus (12 µm) and the smallest mean diameter was studied in Gallus domesticus (10 µm).The largest mean diameter of eosinophil was observed in Corvus splendens splendens (11 µm) and the smallest one was observed in Passer domesticus confucius (10 µm) (Thein H., 1979).The mean diameter of eosinophil of Coturnix japonica (12 µm) was larger than the eosinophil of G.gallus (11 µm) (Term paper, Group XXX VI 2013).In this study, the largest mean diameter of eosinophil was found in Anser cygnoides (12 µm) and the smallest one was found in Gallus domesticus (9 µm).The largest neutrophil were observed in Porphyrio porphyria policephalus (13 µm) and the smallest one was observed in Lonchura punctulata lineoventer (10 µm) (Thein H., 1979).The mean diameter of neutrophil of C.japonica (11 µm) was nearly equal with G.gallus (11 µm) (Term paper, Group XXX VI 2013).In this study, the neutrophils of the collected species were found to have very fine, numerous granules and the nucleus with only lobe. The largest mean diameter was studied in Gallus gallus domesticus (11 µm) and the smallest one was studied in Gallus domesticus (8 µm).The largest mean diameter of lymphocyte was observed in P.porohyrio policephalus (13 µm) and the smallest lymphocyte was observed in Streptophila chinensis tigrina (8 µm) (Thein H., 1979).The mean diameter of lymphocyte (11 µm) and monocyte (11 µm) were nearly equal in studied birds (Khin et al., 2014).The mean diameter of lymphocytes and monocytes were nearly equal in G.gallus and C.japonica (11 µm) (Term paper, Group XXX VI 2013).In this study, the largest mean diameter of lymphocyte was found in Gallus gallus domesticus (10 µm) and the smallest lymphocyte was found in Gallus domesticus (9 µm).Monocytes are found in spleen, red bone marrow and are motile and phagocytic. The monocytes are largest cells along the white blood cells, they are also found in avian blood (Maxwell et al., 1979).In this study, the largest mean diameter of monocyte was studied in G.domesticus (12 µm) and the smallest one was found in Anas platyrhynchos (10 µm).In vertebrates, expect mammals, the blood has thrombocytes or spindle cells, they are small in size with clear cytoplasm and an oval or rounded nucleus, and they help in clotting of blood. Blood regulates and equalizes body temperature by carrying heat away from regions where it is produced (Jordon, 2004).The average diameter of the thrombocytes is 4 µm. The average diameter of the nucleus is 2 µm. (Thein H., 1979).The largest mean diameter (6 µm) and nucleus (5 µm) of thrombocytes was nearly equal in the studied species (Khin et al., 2014).The mean diameter of thrombocyte and was larger in C.japonica (6 µm) with G.gallus (5 µm) and the nucleus of thrombocyte was also larger in C.japonica (5 µm) with G.gallus (4 µm) (Term paper, Group XXX VI 2013).In this study, the largest mean diameter of thrombocyte was found in Anas platyrhynchos (7 µm) and the smallest one was found in G.g.domesticus (6 µm). The mean diameters of nuclei of thrombocytes (5 µm) were equal in studied birds. The freshly a clearly meats and eggs are important for consumption all over the world. Thus, the good and safely poultry breeding may be made for people.
6. Conclusions
- In this study was conducted to determine normal hematological values of blood in the two species (Anas platyrhynchos and Anser cygnoides) of ducks and one species (Gallus domesticus) and one subspecies (Gallus gallus domesticus) of fowls. Blood species from 5 number of each species were used for the determination of hematological indices values. The morphological measures obtained in this study were red blood cells, three granular white blood cells consisting basophil, neutrophil and eosinophil and two non- granular white blood cells consisting lymphocytes and monocytes. However, there were specific variation to cell size between the studied species of ducks and fowls. The consumption of birds especially fowls, duck and their eggs are important for healthy diet. Thus the productions of cleaned and fleshy meats are essential for people. The good poultry farms must be breed in our country. In addition, the observing data from this work would also partially form the basis for further research on other species of blood cells.
ACKNOWLEDGEMENTS
- We are grateful thanks to Dr. Kyaw Kyaw Khaung, Rector, East Yangon University for permission to work this departmental research. We wish to express our greatly indebted to Dr Thet Thet Myaing, Professor, Head of Zoology Department, East Yangon University, for her accepting to work on the present topic, valuable advice, supervisions and kind permission to use facilities available in the Zoology Department. We would like to thank Dr Khin Nang Myint, Professor of Zoology Department, East Yangon University, for her suggestion and advices.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML