-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2021; 10(1): 1-13
doi:10.5923/j.als.20211001.01
Received: Jan. 6, 2021; Accepted: Jan. 30, 2021; Published: Feb. 6, 2021

Update on Aquaporin, Contribution to the Diarrhoea Pathology
1Laboratory of Pathogenic Microbiology and Immunology, Collage of Life Science, Jilin Agricultural University, Xincheng Street, Changchun City, Jilin Province, R.P China
2Ministry of Education, Engineering Research Center for Bioreactor and Pharmaceutical Development, Jilin Agricultural University, Xincheng Street, Changchun City, Jilin Province, R.P China
Correspondence to: Farida Seif, Laboratory of Pathogenic Microbiology and Immunology, Collage of Life Science, Jilin Agricultural University, Xincheng Street, Changchun City, Jilin Province, R.P China.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Emerging evidence acknowledge the view that Aquaporin’s (AQP’s) water channels are the controller of transcellular water flow in the Gastro intestines (GI), relating with their expressions in most intestinal tissues. And diverse physiological and pathophysiological processes. Owing to peculiar role of AQP’s in the GI, Changes in their distribution play important role in etiopathogenesis of diarrhoea, as they have direct involvement in the normal dehydration of faecal contents. Intensive studies on animal and cell model suggested inflammation and regulatory pathways such as cAMP/PKA/CREB affects activities of AQP’s gene expression, and the current information on regulatory pathways and directed mechanistic research are hypothesized to work out new approach for these clinical implications. Researchers are aimed on developing AQP modulators, blockers and inhibitors for therapeutic needs, and better understanding the role of intestinal AQP’s in inter individual susceptibility to diarrhoea diseases. Here we found that, α-ketoglutarate (AKG), nisin, genistein, anti-diarrheal Chinese medicine: Rhubarb tannin extract, N-acetylcysteine (NAC), neutrophil elastase inhibitor sivelestat and deletion of AQP4 help in clinical management of intestinal diarrhoea in different models of diarrhoea. It is there fore effective at reducing symptoms, possibly specific targets for AQP’s modulation. This review seeks to compile scientific evidence on changes in the abundance and modulation of AQP water channels that occur in different models of diarrheoa, marking a potential novel therapeutic target.
Keywords: Intestinal AQP’s Physiology, Diarrhea Pathology, Inflammatory Response, cAMP/PKA/CREB and Therapy
Cite this paper: Farida Seif, Linbo Zhang, Update on Aquaporin, Contribution to the Diarrhoea Pathology, Advances in Life Sciences, Vol. 10 No. 1, 2021, pp. 1-13. doi: 10.5923/j.als.20211001.01.
Article Outline
1. Introduction
- Aquaporins (AQP’s) are fundamental proteins which are classified among the major intrinsic protein family. They form channels in the cell membrane, which regulate water movement and partially allow the movement of ions or other micromolecules in and out of the cells [1-3]. Scholars around 1980s to early 1990s by chance discovered an enormous protein that causes osmotic flux of water in the membrane of red blood cell [4-6]. They were unknown until the first discovery of a 28kDa membrane protein, "Aquaporin-CHIP28", and later known as Aquaporin one (AQP1). Transcripts and proteins related to the AQP-CHIP water channel and their activity was demonstrated by practical work in a Xenopus oocyte. The DNA transfection in oocytes has showed that AQP is the one that permits the diffusion of water molecules through the cell membrane in response to an osmotic reaction. Since, it completely increased the rate of osmotic swelling of oocytes. Isomers of AQP’s, have been categorized into three classes; "orthodox" "classical" Aquaporins, Aquaglyceroporins and Superaquaporins (S-aquaporins) respectively. The orthodox/classical Aquaporin and Aquaglyceroporins discretely which permits movement of water, glycerol, and small solutes. While S-aquaporins only are present in mammals and have an uncertain permeability [7-11]. Currently, fifteen (15) genes encoding AQP’s in Mammals AQP0 - AQP14 [12-15], and in total, thirteen (13) isomers of AQP’s were identified in human body AQP0 - AQP12 [16,17]. The classical AQP’s are AQP 0, AQP 1, AQP 2, AQP 4, AQP 5, AQP 6, and AQP 8. The aquaglyceroporins are AQP3, AQP 7, AQP 9, and AQP 10 [18-20]. And finally, Mammalian S-aquaporins are AQP11 and 12 [11,20,21]. Different subfamilies of AQP’s they are located and expressed at membrane sites in most epithelial of different tissues [22-25]. Even if the functions of AQP’s are well described in the kidney, lung, and heart, the knowledge on the subject of AQP’s pathophysiologic functions in the gastrointestinal tract remains to be illuminated. This paper aimed at reviewing the pathophysiology of the potential role of AQP’s in Gastro intestine (GI) specific to small and large intestine in different diarrhoeal pathological conditions.
2. The AQP’s Dynamic Physiology, and Their Expressions and Distributions in Normal Human’s Small and Large Intestine
- The physiologic role of the GI is complicated metabolic processes associated with the small intestine and colon as well as other primary organs. Along the GI wall, the existence of various specialized differentiated cells and gut microbiota permeates severus processes with the last act in the production of feces. Immediately after food intake, nutrient and water, came to be drawn up in the first segment of the intestinal tract, this stabilise osmotic pressure on the GI content and uplifted fluid recirculated within the intestinal cells. The fluid absorptions within the small intestine and the colon is ant osmotic and isotonic respectively (the balance is regulated with the Gl hormone, neurotransmitter, inflammatory mediators and AQP’s). The AQP’s is important modulator in fluid transportation in GI tract. [26]. Most essential for near-iso osmolar trans-epithelial rapid fluid movement driven by pressure gradient [27-29]. Their appearance in a variant forms distributed all over the gut has been sufficiently shown [30]. Among thirteen (13) AQP isoforms that is expressed in humans, at least eleven (11) AQP isomers located in the GI epithelial cells [31], with AQP1, AQP2, AQP3, AQP4, AQP7, AQP8, and AQP10 suggested to play are critical function’s in the GI (large and small intestine) related to intestinal fluid absorption and secretion. More ever, extensive evidence suggested most of the GI AQP’s were localized within the salivary gland, stomach, small intestine, gallbladder, pancreas, and finally, the colon [32,33], providing clues to their possible functional roles, which in most cases they are localized in cells and particularly tissues required higher water permeability [34].
2.1. Gastro Intestinal AQP’s in Relation with Absorption and Digestive Function
2.1.1. The Physiologic Role’s of AQP’s and their Expression in the Human Small Intestine
- The lining of the small intestinal cells are classified into two types: the villus enterocytes and crypt cells. The villus enterocytes are the cells covering the villi which is matured and non-proliferative cells differentiated to perform the role of digestion and absorption.The expression and tissue distribution of AQP’s in the small intestines, are largely connecting AQP’s with the enterocyte for the fast-bidirectional movement of fluid, which is related to both and secretory and absorptive processes. The most common AQP’s isoforms that have been identified in human small intestine are AQP1, AQP3, AQP7, AQP8, and AQP10 (See Figure 1) mostly presented in the crypt of enterocytes and superficial villi, which revealed their physiologic role in fluid and solute secretion and absorption. The detection of human AQP3 at basal and lateral surface of villi, goblet cells, granule containing cells, and Paneth cells indicates the AQP3 physiologic role in fluid reabsorption. Meanwhile, both protein levels and mRNA for AQP7 and AQP8 were exclusively presented in the mucosal epithelium surface of human ileum [31,35]. With the cellular localization of AQP8 at apical surface of epithelium of the ileum, and AQP7 along the basal and lateral parts at epithelia of the human ileum, indicating both AQP7 and AQP8 with function’s in fluid reabsorption [36]. Interesting evidence comes with localization of AQP9 and AQP10, both have been detected on mucus secreting goblet cells, first indicating unique function of AQP9 in mucus secretion which protects the small intestinal cells [37], than the distribution of the AQP10 at apical surface domain of goblet cells, at the surface of absorptive cells, and the lining of the villi in the human small intestine, suggested in the small intestine, AQP10 as it could be a main water channel for water transportation [38]. With a suggestion from previous study that water movement occurred from the apical membrane of the absorptive epithelium mainly through AQP10 and partly through AQP8, and across the basolateral membrane through AQP3 [38,39].
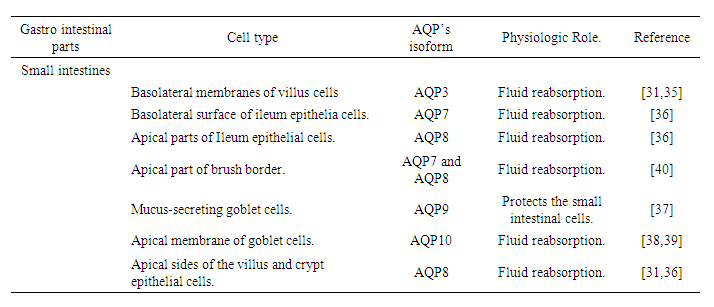 | Figure 1. The AQP’s Cellular Localization along The Human Small Intestine |
2.1.2. The Expression and Physiologic Role’s of AQP’s in the Human Large Intestine
- AQP’s in the colon appeared with their critical roles in many GI studies. It is clear that the absorption’s of water in the colon draws approximately 1.5 to 2 litres of water per day across an osmotic gradient with outermost movement predominantly occurring through AQP’s. Data indicated AQP1, AQP3, AQP4, AQP7 and AQP8 as the principal AQP’s in the human colon. AQP1 was located in the human colonic vascular endothelium of mucosa cells [41,42], the AQP2 is proposed to functions in colonic water absorption [43] since they are expressed along the membrane of absorptive surface in the human colonic cells [44]. The expression AQP3 is at apical surface of human villus colonic cells, the AQP3 in the colon seems to be of special interest [34]. Silberstein and his group [45] for the first time have identified functional AQP3 in the human colon suggested with a role in fluid absorption across this particular intestinal section. The fact that the intercellular junctions of colonic cells were reported to be tight limits water transport across the paracellular route and suggests the transcellular route as the main pathway for water movement driven by ion absorption. If this is the case, regulation of AQP3 may contribute to maintaining the control of water absorption and its alteration could lead to abnormal states such as constipation and diarrhoea, with inhibition of AQP3 function either by Mercury(II)chloride (HgCl2) or Cupper(II)Sulphate (CuSO4) was reported to causes diarrhoea [45,46]. Meanwhile, the detection of AQP4 was at basal and lateral surface of colonic cells, Ishibashi and his colleague [19] denote involvement of AQP4 in the dehydration of the fecal contents through rapid restoration of luminal water back into the body [19], with a former literature revealed a higer content water in feces and reduction in osmotic water permeability in AQP4-/- knock out mice [20]. Additionally, the detection of AQP7 was at basal and lateral part of in the crypt and villi epithelia of human colon [36] while AQP8 was detected along the apical sides of the villus and crypt epithelial cells in the human colon, which indicated their possible roles in the secretion or absorption of water at these sites [31,36]. In general, AQP3, AQP4, AQP7, and AQP8 play remarkable roles in controlling GI fluid balance within the colon. For instance, the inhibition of both mRNA and their protein expression of gastric AQP3 and AQP4 by exposure to mercury, as well as protein expression of AQP3 and AQP7 in the in the small and large intestines of rats, led to the accumulation of intestinal fluids and, finally, causes diarrhoea [31,47].
3. The AQP’s Alteration and Their Involvement in the Diarrhoea Pathology
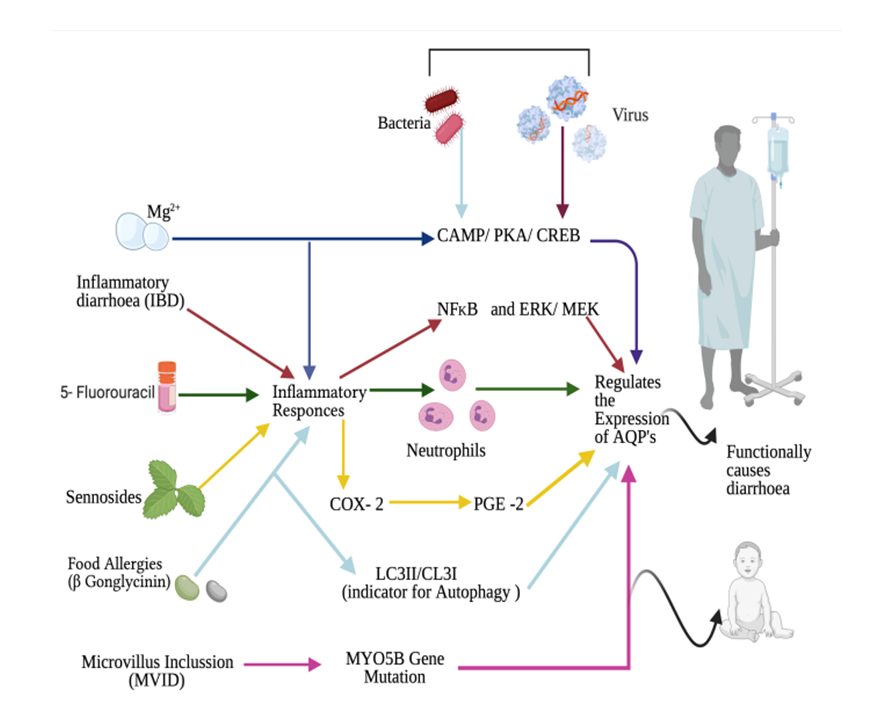
- Diarrhoea, is identified with recurrent and watery bowel movements, generally resulted by GI infections (see in Figure 4), diet changes or other illnesses (See in Figure 5). Studies indicated changes in the expression or interrupted function of AQP’s influence the transportation of water (See in Figure 3). And the interaction between dysregulated AQP’s and diarrhoea has also been related in many diarrhoea diseases.
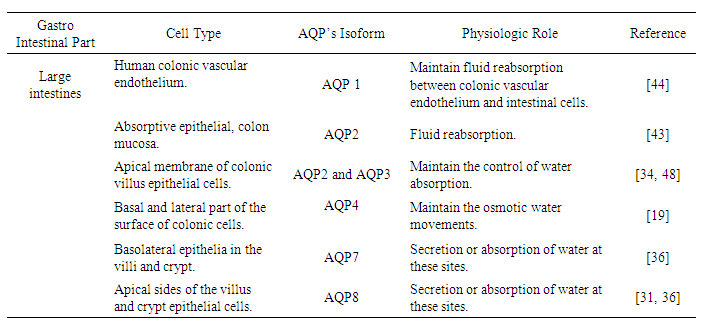 | Figure 2. The AQP’s Cellular Localization along The Human Large Intestine |
 | Figure 3. Different Diarrhoea Models Depicting the Pathologic Mechanisms Involving AQP’s Alteration / Regulation |
 | Figure 4. The Dynamic Regulation of AQP’s Expression in Infectious Diarrheal |
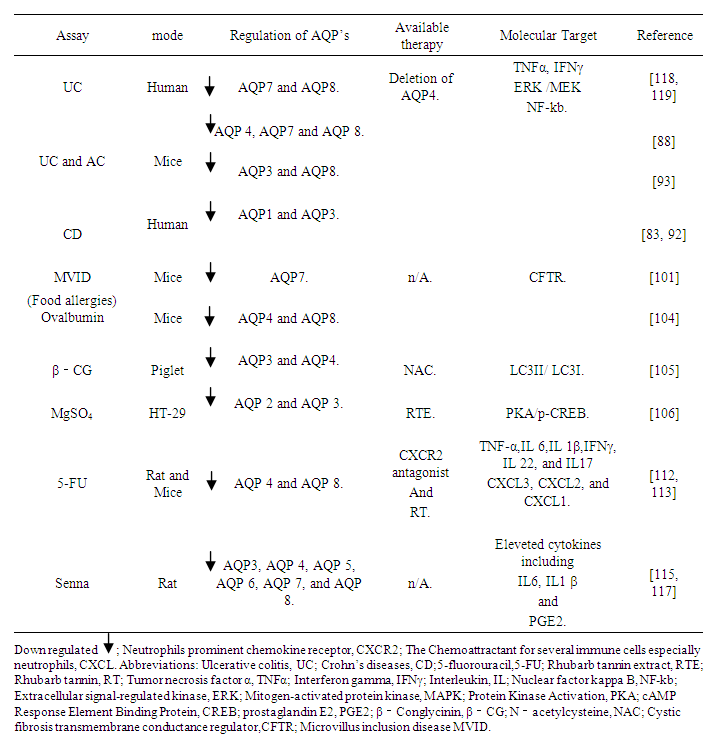 | Figure 5. The Dynamic Regulation of AQP’s Expression in IBD, Chemotherapy treatments (5-FU) and Laxative effects of MgSO4 and Senna products in their contribution of Diarrhoea |
3.1. Physiologic Role of AQP’s in Pathogenesis of Diarrhoea Induced by Bacterial Infections
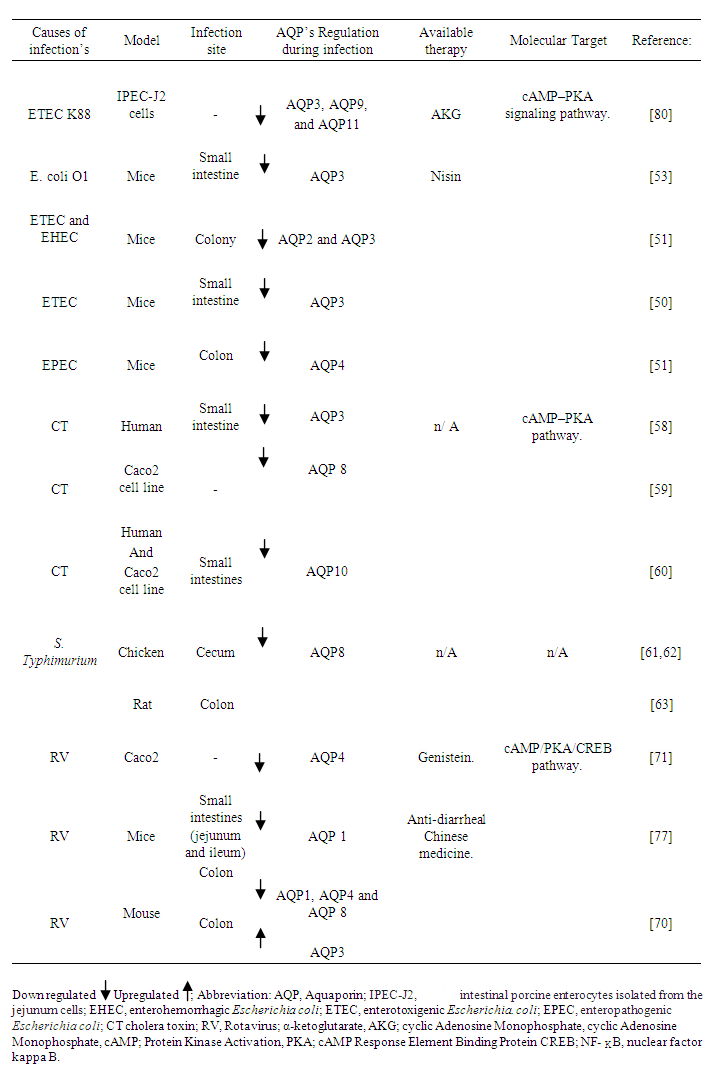
- Infectious bacterial diarrhoea is always correlated with the rapid deprivation of intestinal fluids and electrolytes characterized with either a sharp rise in intestinal secretion and/or a decline in intestinal absorption [49]. In multiple diarrhoea model, infections causes changes in AQP’s distribution thus play important role in etiopathogenesis of diarrhoea.For example, the mechanism which enterotoxigenic Escherichia coli (ETEC) induced diarrhoea is through, their localization action to the small intestine and release of enterotoxin to the intestinal cells, which further drives the secretion of intestinal fluid. The first report to demonstrates ETEC induced diarrhoea is involved with defects in water reabsorption within the jejunum of the small intestines was connected to reduction of AQP3 water channel [50]. While diarrhoea infection caused by Enterohemorrhagic Escherichia coli (EHEC) and ETEC, reduced both AQP3 and AQP 2 in the colony of mice [51]. Moreover, Zhang Di [52] showed possible mechanisms of diarrhoea which is correlated with the rapid decrease in both protein level and mRNA expression of AQP4 in the ileum followed by Enteropathogenic Escherichia coli (EPEC) infection, and pathogenic infections of E. coli O1 also reduced expression of AQP3 [53]. Additionally, up regulation of AQP3, AQP 4, and AQP 8 from diarrhoea infection caused by E. coli (liposaccharide (LPS)), suggesting an α-ketoglutarate (AKG), can likely be equally used as an emerging therapy for diarrhoea treatment through regulatory mechanisms of AMP-activated protein kinase (AMPK) pathway marked with the reduction of nuclear factor kappa B pathway (NF-κB) [54]. On the other hand, nisin influence increases the expression of AQP 3 in the jejunum, colon, and duodenum, specifically in the jejunum. AQP3 functions in the inhibition of diarrhoea influence rapid recovery of intestinal fluid [55].The Gram-negative bacteria Vibrio cholerae are the main causes of cholera which is associated with serious watery diarrhoea. The main virulence factor of V. cholerae is Cholera toxin (CT), consisting of an enzymatic (A) subunit and 5 binding (B) subunits. Cholera toxin acts by the following mechanism: First, the binding of its B subunit to GM1 ganglioside receptors located in the apical membrane of intestinal epithelial cells (IEC), CT is internalized and the CT A subunit is released into the cytosol, where it induces intracellular cyclic Adenosine Monophosphate (cAMP) generation, enabling cAMP-mediated intestinal fluid secretion [56-58]. Former evidence demonstrated a changes in water permeability happened with downregulation of AQP3 followed by CT [58]. While, observations in the rat intestine also verified the down-regulation of AQP8 [59], and AQP10 in human cholera patients [60]. The altered AQP’s regulation in CT induced diarrhoea has been linked to increased intracellular cAMP concentration. The possibility that these AQP’s are responsible molecules for causing acute secretory diarrhoea as in cholera was considered, with an adenylyl cyclase (AC) accelerator forskolin showing similar effects as CT. However, further investigation is needed to identify what mechanism lays between the increase of cAMP concentration and the changes of water permeability of AQP’s. Like wise S. Typhimurium downregulate the AQP8 expression in the colon of rats and caecum of chicken [61,62]. However, mechanisms of regulation and information regarding other isomers of AQP's and their direct involvement with S. enterica serovars are still to be researched, perhaps new research could disclose a new scope in understanding cellular susceptibility to pathologies caused by Salmonella enteritidis serovars. Meanwhile, diets supplemented with prebiotics such as fructo-oligosaccharides (FOS), lactulose, and inulin consistently increased intestinal Salmonella translocation in rats and they have been associated with the increase in AQP3 [63].
3.2. Physiologic Role of AQP’s in Pathogenesis of Diarrhoeas Induced by Viral Infection’s
- Viruses are the most common etiologic agents, which is the most frequent pathogens associated with diarrhoea. Pathogenic virus example Rotavirus (RV) is a worldwide main cause of infantile diarrhoea and gastroenteritis. Clinical studies have showed, RV infection is actively related to symptoms, such as abdominal pain, fever, vomiting, acute gastroenteritis, dehydration, and diarrhoea [64]. RV diarrhoea has been connected to several distinct mechanisms, including a virus-encoded toxin, malabsorption secondary to enterocyte destruction, villus ischemia, and stimulation of the enteric nervous system (ENS) [65,66]. Over the past decades, diverse studies has conveyed the mechanisms of diarrhoea initiation at the tissue and cellular levels, and new knowledge is beginning to emerge. The diarrhoea is regarded to be secondary malabsorptive to enterocyte destruction [66,67]. In a way that the secretion of excess fluid largely resulted from active chloride emanation to the intestinal lumen, which causes minor movement of water and sodium. During this process, it is supposed that RV secreted a viral enterotoxins named nonstructural protein (NSP4), stimulate calcium-activated chloride channel(s) (CaCCs) while block sodium ion (Na+) or glucose cotransporter (SGLT1) across the enterocytes luminal membrane [68,69]. At present, several Na+ and chloride ion (Cl-)channels have been identified in RV cause secretory diarrhoea: sodium potassium chloride cotransporter (NKCC1), epithelial Na+ channel (ENaC) and TMEM16A. However, the expression and pathophysiological role of water channel AQP’s in RV infection has yet to be fully established [70-72]. Literatures suggested RV NSP4 might increase the cAMP concentration by attaching to specified receptors within intestinal cells, resulting in the secretion of Cl- and further reducing the absorption of sodium and water [73,74]. In the same way, activity of AQP’s is notably increased with protein kinase A (PKA) activators such as cAMP and forskolin [75,76]. Meaning that, a cAMP/PKA/ cAMP- Response Element Binding Protein(CREB) are possible signalling molecules linking the AQP’s and RV diarrhoea pathology, as genistein provided new anti-RV strategy by inhibiting rotavirus replication and upregulating AQP4 expression via the cAMP/PKA/CREB signaling pathway [71]. These findings suggest the pathophysiological mechanism of RV infection involves decreased expression of AQP’s. In line with what have been said above, Cao and his colleagues [70] observed gradually decreased of protein expression levels AQP 1 in the ileum, and AQP1, AQP4, and AQP8 in the colon followed by RV infection’s. Chen H, et al. [77] reported infection by RV-SA-11 decreased AQP1 mRNA in a mouse model of diarrhoea, and the treatment with antidiarrheal oral liquid traditional Chinese medicine, in turn upregulated AQP1 mRNA while ameliorating the effects of diarrhoea. AQP1 are said to be localized at colonic vascular endothelium cells [41,42], this further suggesting the decrease in AQP1 inhibited water transfer from the intestinal tract to the vascular side and causes diarrhoea.Furthermore, AQP4 and AQP8 are an important modulators of intestinal fluid movement in the colon. As previously suggested, deletion of AQP4 gene in a mice demonstrates higher water content of defecated stool [70,78], and small interfering RNA notable reduce absorption of water in rat colon through direct inhibition AQP8 [79]. On the contrary, protein expression of AQP3 in the colon was drastically increased in a mice infected RV [70], suggested was the balancing mechanism toward avoiding severe diarrhea and further dehydration.
3.3. The Roles of AQP’s in Inflammatory Bowel Disease, Diarrhoea, Pathological Mechanism
- Crohn’s disease (CD) and ulcerative colitis (UC) are two forms of IBD that are related to reduced fluid intake with impaired secretion, causing a defect in barrier function marked with the involvement of diarrhoea [81]. The pathogenesis of IBD is still uncertain, although different attributes of GI immune system take part in IBD etiology [82]. Ricanek et al. [83] showed the mRNA expressions of AQP 7 and AQP 8 were downregulated in the early stage of patients with UC, with significant decrease in both, AQP1 and AQP3 mRNA levels were in the ileum of patients with CD. The AQP1 protein was downregulated in HIMECs after coculture with C. difficile with related IBD symptoms, suggesting C. difficile influenced the occurrence and progression of diarrhoea by suppressing AQP1 protein expression and inhibiting intestinal cell permeability [84]. Furthermore, depicted loss of AQP1, AQP3 and AQP 8 in the intestinal cell in CD patients and distinct apical localization (migration) has been witnessed in several studies. The loss of apical polarization, and reduction of, AQP1 and AQP3 mRNA levels were observed in ileum of patients with CD [83], and reduction of AQP8 mRNA in the apical area of colonic surface in CD [36]. The reduction’s and cellular migration serve as an indicator for disturbance in epithelial polarity, resulting in GI barrier injury and malfunction [85,86].The description on the potential functions of AQP’s in all forms of IBDs (CD and UC) showed distinctive distribution pattern in the GI and the interaction between GI inflammation and the physiologic in water and ions trafficking and adjustment has also been established [87]. Former study highlighted the change in AQP’s, suggesting dextran sodium sulfate (DSS) caused colonic injury through downregulation of AQP’s; the model demonstrated that colitis disease is linked with Th1 immune response same as in human UC. In the acute phase, tumor necrosis factor α (TNFα), interferon gamma (IFNγ), Interleukin 1 (IL 1), and IL 12 all upregulated, followed by reduction of AQP 4, AQP 7, AQP 8 mRNA levels [88]. Ifnγ behaves in various ways to influence inflammation, for example, increasing the secretion of a proinflammatory arbitrators by activating, manipulating the cellular trafficking process by controlling the characteristics of the adhesion molecules, and changes in the intestinal epithelial barrier permeability [89,90]. In the same way, TNF-α are considered as a pleiotropic cytokine which evokes a broad spectrum pathogenic as well as physiologic process [91]. Its cause effect in many pathological disease conditions and control the expression AQP’s, for example, TNF-α upregulated in the feces and serum of patients with CD, consequently downregulates AQP1 and AQP 3 mRNA in the ileum [83,92]. Although, in the trinitrobenzene sulfonic acid (TNBS) model of colitis, TNFα may also causes an unevenness in regulatory cytokines, which finally resulting in a remarkable reduction of both AQP 3 and AQP 8 protein and mRNA expression [93]. However, scholars indicated the mechanisms that both IFNγ and TNFα synergized to induce intestinal epithelial barrier dysfunction. Recent work showed that IFNγ and TNFα are capable of downregulation AQP's [81]. IFNγ downregulates AQP3 mRNA, and these activities are depending on an activator of transcription (STAT 1) and signal transducer [64,78]. In contrast, Peplowski [94-96] demonstrated in HT-29 human colon cancer cells line, TNF α down-regulate AQP3 expression with increasing activity of transcription specificity protein (Sp3). However, this action might be interrupted with inhibition of NF-kb and extracellular signal-regulated kinase/ mitogen activated extracellular signal-regulated kinase (ERK /MEK) pathway. Since the NF-κB has been reported to be involved in different kinds of diarrhea, NF-κB activation has been important to the downregulation of the AQP3. Additional studies confirmed, the effective blocking AQP4 may represent a novel therapeutic approach for UC since knockdown AQP4−/− mice showed higher IL-10 level and lower levels of TNFα and IL-6, and lesser inflammatory cell infiltration also, NF-κB p65 as well as nuclear levels of p65 and phosphorylated p65 [97]. These findings imply that inflammatory cytokines regulate missing localization and downregulation of AQP, and their effects impair the ion's channels and lead to the pathogenesis of IBD [92]. Therefore, the fluid metabolic anomalies and alteration in GI permeability would be the performance of IBD by downregulating AQP 3, AQP 4, AQP 7, and AQP8.
3.4. AQP’s Physiologic Roles in Pathogenesis of Diarrhoea Associated with Microvillus Inclusion Disease (MVID)
- Microvillus inclusion disease (MVID) is a rare congenital disorder characterized by blunted or absent microvilli with accumulation of secretory granules and inclusion bodies in enterocytes [98]. A typical clinical presentation of this disease is chronic secretory diarrhea, nutrient malabsorption and death in infants. Emerging evidence revealed mutations in MYO5B as a cause for MVID, and has paved a new way to understand the pathogenesis of this disease [98,99]. Weis et al. [100] described the deletion of the MYO5B gene in mice and its close phenotypic similarity to the human disease with evident microvillus inclusions and loss of apical transporters in the duodenum. However, it is a controversy whether knockout of MYO5B resemble the presence of a mutated MYO5B protein since these mice did not show an intestinal barrier defect [101], compared with MVID patient [98,102]. MYO5B regulate trafficking of many apical transporters. However, trafficking the of Cystic fibrosis transmembrane conductance regulator (CFTR) is largely independent of MYO5B. And tamoxifen-inducible VilCreERT2; MYO5Bflox/flox model demonstrated decreased in expression of several apical transporters including AQP 7, with preservation CFTR. Although the decreased in apical localization AQP7 and other transporters thought to induce dysfunctional water absorption in enterocytes of patients with MVID. Maintaining CFTR exacerbate water loss by active secretion of chloride into the intestinal lumen [101].
3.5. AQP’s Physiologic Roles in Pathogenesis of Diarrhoea Associated with Diarrhoea Induced by Food allergies
- Food allergies have become increasingly prevalent during the past few decades, affecting up to 6% of young children and up to 4% of adults [103,104]. Diarrhoea is one of the most frequent intestinal symptoms caused by food allergens. Despite the scarce study focusing on AQP’s association and food allergies. Existing literature pointed allergic diarrhoea is connected with downregulation of AQP4 and AQP8 mRNA levels in the proximal colon mice [104]. Make it possible to suggest that, these reductions resulted in the dysfunction on the water absorption in the proximal colon and then caused diarrhoea to wash out the food allergen. Furthermore, an anti-nutritional factor β-Conglycinin (β-CG) activate autophagy and induce intestinal dysfunction and diarrhoea via downregulation of AQP3, AQP4. Meanwhile, pre-treating the β-CG challenge piglet with N-acetylcysteine (NAC) decreased Atg5 protein abundance and the LC3II/ LC3I ratio (an indicator of autophagy) improved intestinal function through upregulations of intestinal transporter protein including AQP3, AQP4 and attenuated intestinal autophagy in β-CG- challenged piglets [105].
3.6. Physiologic Roles of AQP’s in Pathogenesis of Diarrhoea Induced by Magnesium Sulphate
- Magnesium sulphate (MgSO4), as an osmotic acting laxative, introduced to induce diarrhoea by rising intestinal contents along with the inhibition of water reabsorption. The laxative effects of MgSO4 upregulates expression of AQP3 in HT-29 cells through the PKA/p-CREB signal pathway [106]. As in HT-29 cells, natriuretic peptide and vasoactive intestinal polypeptides are associated with triggering the stimulation of PKA, which is connected with increased AQP3 expression [107,108]. Similar to that, Rhubarb tannin extract (RTE), which is a traditional antidiarrheal Chinese herbal medicine, significantly attenuated MgSO4 induced diarrhoea in a dose dependent manner in HT-29 cells, suggested by the RTE inhibitory effect on AQP 2 and AQP 3 is partly done by the downregulation PKA/p-CREB signal pathway [106].
3.7. Physiologic Roles of AQP’s in Pathogenesis of Diarrhoea Induced by 5 - Fluorouracil
- The chemotherapy drugs, 5-fluorouracil (5-FU) are most useful in treating various cancers, including breast cancers and colorectal cancer [109]. Diarrhoea commonly happen as side effect encountered with cancer patients going through clinical chemotherapy, essentially with 5-FU. The enterocytes apoptosis showed a dose of 5-FU induced direct toxic effects such as; blocking of the synthesis of deoxyribose nucleic acid (DNA) and inhibition of the production of subsequent oxidative stress (OS) with reactive oxygen species (ROS) and induction of inflammatory response. Both TNFα and OS are said to induce tissue inflammatory response, TNFα also activates the up-regulation of NF-κB and its translocation into the nucleus [110-112]. TNF-α, IL 6, IL 1β, IFNγ, IL 22, and IL17 have been involved in 5-FU induce diarrhoea. However, it was not clear whether 5-FU induces inflammation cause changes in AQP’s, Sakai [113] reported the treatment with 5-FU significant increased inflammatory cytokines, on the contrary, the genes for AQP 4 and AQP 8 were remarkably decreased. Assumed with AQP 4, downregulation happened as a result specific to either enterocytes damaged and/or inflammatory response induced by chemotherapy [112], with TNF-α overexpression reported with no effect on impaired AQP’s however it dramatically influences several inflammatory cytokines [113]. On the other hand, significant increased expression levels of CXCL3, CXCL2, and CXCL1 with the neutrophil markers Elane and Myeloperoxidase (MPO), correlated with the decreased expression levels of AQP 4 and AQP 8 in the colon [114]. While the use of CXCR2 antagonist SB225002 or preadministration of either the neutrophil elastase inhibitor sivelestat sodium decreased neutrophil release induced 5-FU in the colon and increases the expression of AQP 4 and AQP 8 pointing a potential therapeutic target. Moreover, Rhubarb (Rh), provided the alternative therapy, dose dependent efficacy of RE described with the capability to decrease severe ileal mucositis through downregulation of matrix metalloproteinase 9 (MMP-9), TNF-α, NF-κB and upregulation AQP4 [112].
3.8. Physiologic Roles of AQP’s in Pathogenesis of Diarrheal Induced by Senna, Sennosides, and Sennoside A
- Senna which is known as Cassia angustifolia Vahl or Cassis acutifolia Delile and its main ingredients sennosides linked with anthranoid glycosides that contains a different anthraquinone derivatives such as sennoside A, B, C, and D.They are commonly used as potential laxative drugs in the treatment of intestinal constipation. Senna has been connected with regulatory functions of water transport in the colon through AQP’s. Being a laxative drug, senna products are said to stimulate the increases in fecal water content, motility of feces, and peristalsis over the colon. Consequences has been demonstrated by the former studies with the suggestion that diarrhoea might be generated even with small administration of sennosides. The Sennoside administration confirmed with regulation on numerous expressions of AQP’s water channel, substantial down regulation was found on water specific AQP’s including AQP 4, AQP 5, AQP 6, AQP 7, and AQP 8 [115]. Meanwhile, the laxative effects of sennoside were also connected with a decrease expression of AQP3, which is thought to be induced by inflammatory responses via activated macrophages [92,94,116]. However, this response was attenuated with the long-term administration, of Kanzo ingredient known as glycyrrhiza, with the combination of sennoside A. In addition to that, gut microbiota homeostasis might be involved in regulating AQP3 expression [116], Kon [117] reported both Sennoside A and B, are metabolized by intestinal bacteria into active metabolite rheinanthrone. Rheinanthrone role is to increase expression of cyclooxegenase 2 (COX2) within the macrophage resulting in dramatic increase in prostaglandin E2 (PGE2) expression, raises in PGE2 followed with downregulation in expression of AQP3. A suggestion that PGE2 might be involved in the drastic and immediate decrease in AQP3 in the mucosal epithelial cells in the colon that was induced by sennoside A. Pre-treatment with indomethacin COX inhibitor decreased PGE2, on the other hand, neither decreased the AQP3 expression nor induced diarrhea, reduction of AQP3 expression alone likely thought to produces the laxative effect by restricting water reabsorption by the large intestine thereby increasing fecal water content.
4. Conclusions
- The recognition of AQP’s evoked many researches on their expression and functions. In this review, we analyse AQP’s expression and their association and diarrhoea, and we examine AQP’s role in pathological condition’s related to diarrhoea disease. The interaction between AQP's, inflammation and diarrhoea, suggested AQP’s may be used as promising therapeutic target in treatment and prevention of diarrhoea in both humans and animals. The existed information is able to tell us AQP are regulated in different diarrhoea models. Analyzing the patterns and mechanisms of regulation will perhaps provide further ideas to research and disclose a new scope in understanding cellular susceptibility to pathologies of enteric diarrhoea. Future research should focus on clarifying mechanisms that cause alterations in AQP’s expression during diarrhoea pathogenesis, investigating the process and moderator causing the AQP’s regulation, and what changes body homeostasis is needed in order to effective design an appropriate therapeutic target. However, some existing studies suggest that AQP’s stabilization through transcriptional gene regulation and the use of probiotics present research significance and could have been an effective therapeutic approaches in the treatment of diarrhoea.
Data Availability
- No data were used to support this study, all references were dully acknowledged, while scientific illustration ware Created with BioRender.com.
ACKNOWLEDGEMENTS
- I thank the Jilin Province Science and Technology Development Plan Project for supporting my research and the Laboratory of Pathogenic Microbiology and Immunology, Collage of Life Science for helpful discussion and valuable comments.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML