-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2018; 8(2): 51-56
doi:10.5923/j.als.20180802.02

Improving the Quality of Jasminum grandiflorum Flower Buds by Delaying of Senescence
Mohanasundari P.1, T. Sivakumar1, K. Krishna Surendar1, M. Ganga2
1Department of Crop Physiology, TNAU, Coimbatore, India
2Associate Professor (Horticulture) and Head, TNAU- HRS, Ooty, India
Correspondence to: Mohanasundari P., Department of Crop Physiology, TNAU, Coimbatore, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An experiment was conducted to study the flower senescence physiology in Jasminum grandiflorum. Hence, the senescence is a highly coordinated complex and genetically programmed natural process that mainly regulated by phytohormones. The experiment was conducted by following factorial completely randomized design with three replications. The harvested jasmine flowers were treated with different anti-senescence chemicals viz., Silver nanoparticle (20ppm), Boric acid (4%), sucrose (4%), NAA (100 ppm), BA (500 ppm), α-AIB (20µM) and packed in 200 gauge with no ventilation then stored in cold storage and ambient storage condition. Different physiological and biochemical parameters were taken up at three different stages like bud stage, open stage and senescence stage. Among these treatments silver nanoparticles (20 ppm) shows positive significant difference in moisture content, membrane stability index, protein content, phenol content and improve the flower bud quality over the control.
Keywords: Jasminum grandiflorum, Senescence, Storage, Shelf life
Cite this paper: Mohanasundari P., T. Sivakumar, K. Krishna Surendar, M. Ganga, Improving the Quality of Jasminum grandiflorum Flower Buds by Delaying of Senescence, Advances in Life Sciences, Vol. 8 No. 2, 2018, pp. 51-56. doi: 10.5923/j.als.20180802.02.
Article Outline
1. Introduction
- The demand for the jasmine flowers increased in India as well as some of neighbouring countries like, Singapore, Malaysia, Sri Lanka and the Middle East countries but also to distant nations including the United States. J.grandiflorum belongs to the family “Oleaceae” and it is derived from an Arabic word “Jessamine” (Bailey, 1947).The senescence of the flower is a complex process and act as an excellent model system for study of senescence while compared with other parts of plant organ. Flower senescence is the highly genetically regulated or genetically programmed process that involves structural, biochemical and molecular changes to programmed cell death (Yamada et al., 2009, Shahri and Tahir, 2010a).The biotic and abiotic stress like light, temperature, nutrients, ethylene, pathogen attack and pollination etc. accelerated the senescence (Shahri and Tahir, 2010b). Senescence cause shut down of several biosynthetic pathway and the expression of different hydrolases that hydrolyses polymers such as carbohydrates, proteins, lipids and nucleic acids in additional with complex interplay role of different hormones, for example during senescence ethylene and ABA concentration will get increased while the application of cytokinins decrease these two hormone activity. The mechanism of these hormones or their precursors are rapidly transmitted and sensed to bring about the changes in different tissues such as petal, stamens and ovary are not yet well known (Rogers Hilary, 2006). All these changes hastened the senescence process in jasmine flowers.The jasmine flower needs to the local market as well as export are yet to be fulfilled even after the cultivated areas increased year by year due to short shelf life. Hence, the Post harvest physiology of Jasmine is very important. Keeping in view of these constraint present studies entitled on “Improving the quality of Jasminum grandiflorum Flower Buds by Delaying of Senescence” was undertaken at the Department of Crop Physiology of the AC & RI of TNAU, Coimbatore, with the objective, to study the physiological and biochemical role of anti-senescence treatment in flower senescence of Jasminum grandiflorum.
2. Materials and Methods
- The experiment was carried out at the department of Crop Physiology, Tamil Nadu Agricultural University, Coimbatore. The experiment design followed for this study is FCRD and each treatment replicated as thrice. The treatments comprised of six anti-senescence chemicals viz., Silver Nano Particle (20 ppm), Boric acid (4%), sucrose (4%), NAA (100 ppm), BA (500 ppm), α-AIB (20µM) and control. The flowers were harvested at morning time between 6.30 to 7.30am. These flower buds are then immersed in chemical solution by quick dipping method and surface drying was done. After that it was packed in 200 gauge polyethylene bag with no ventilation then stored in cold storage and ambient storage condition. Different physiological and biochemical parameters were taken up at three different stages like bud stage, open stage and senescence stage.The physio-biochemical parameters viz., moisture content, membrane stability index, protein content and phenol content were taken up at three stages of flower bud viz., bud stage, open stage, senescence stage.Moisture ContentThe moisture content of the whole flower was estimated (Barrs and Weatherley, 1962) after recording fresh weight and dry weight of flower buds (Kept in hot air oven at 70°C). Moisture content was expressed in fresh weight basis in percentage and mean of replicated packages was calculated from the following formula,
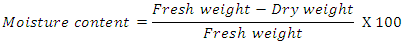 Membrane Stability Index (MSI)Membrane stability index was determined according to the method of Premchand et al. (1990) as modified by Deshmukh et al. (1991). Floret bits (10g) of uniform size were taken in test tubes containing 10 ml of double distilled water in two sets. Test tube in one set were kept at 40°C in a water bath for 30 min and electrical conductivity of the water containing the sample was measured (C1) using a conductivity meter (Henna instrument, HI 2300 EC/TDS/NaCl meter). Test tubes in the other set incubated at 100°C in the boiling water bath for 15 min and their electrical conductivity was measured as above (C2). MSI was calculated using the formula given below:
Membrane Stability Index (MSI)Membrane stability index was determined according to the method of Premchand et al. (1990) as modified by Deshmukh et al. (1991). Floret bits (10g) of uniform size were taken in test tubes containing 10 ml of double distilled water in two sets. Test tube in one set were kept at 40°C in a water bath for 30 min and electrical conductivity of the water containing the sample was measured (C1) using a conductivity meter (Henna instrument, HI 2300 EC/TDS/NaCl meter). Test tubes in the other set incubated at 100°C in the boiling water bath for 15 min and their electrical conductivity was measured as above (C2). MSI was calculated using the formula given below: 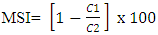 Protein contentFlower petal samples (0.5 g fresh weight) were homogenates with 5 mL distilled water (repeated twice). The mixture was collected and then centrifuged at 4000 rpm for 10 min and the supernatant was collected to determine the contents of soluble proteins by the Coomassie Brilliant Blue G-250 method (Lowry et al. 1951) with bovine serum albumin as the standard.Total Phenol contentPhenol content of the flowers was estimated as per Malick and Singh (1980). Phenols were extracted using 80% ethanol, evaporated to dryness and the development of blue colour by the FC (Folin - Ciocalteau) reagent was measured at 690 nm and expressed as µg equivalent of Pyro-catechol /g of sample.Statistical analysisData collected for protein content and phenol content were directly run by using SPSS 16.0 package. Percentage data of moisture content and membrane stability index were transformed using arcsine transformation before the data analysis. Standard error of difference (S.Ed.) at 5% Probability level used to separate the mean.
Protein contentFlower petal samples (0.5 g fresh weight) were homogenates with 5 mL distilled water (repeated twice). The mixture was collected and then centrifuged at 4000 rpm for 10 min and the supernatant was collected to determine the contents of soluble proteins by the Coomassie Brilliant Blue G-250 method (Lowry et al. 1951) with bovine serum albumin as the standard.Total Phenol contentPhenol content of the flowers was estimated as per Malick and Singh (1980). Phenols were extracted using 80% ethanol, evaporated to dryness and the development of blue colour by the FC (Folin - Ciocalteau) reagent was measured at 690 nm and expressed as µg equivalent of Pyro-catechol /g of sample.Statistical analysisData collected for protein content and phenol content were directly run by using SPSS 16.0 package. Percentage data of moisture content and membrane stability index were transformed using arcsine transformation before the data analysis. Standard error of difference (S.Ed.) at 5% Probability level used to separate the mean.3. Results and Discussion
- Physio –biochemical parameters recorded at bud stage, open stage and senescence stage. Significant difference was observed at all the three stages in both ambient and cold storage condition. In the entire recorded parameters silver nano particle (20 ppm), maintain height values in cold storage condition and NAA maintain highest values in case of ambient storage conditions.
3.1. Effect of Treatments Storage Condition on Moisture Content
- There is a significant difference observed among the treatments in moisture content at open and senescence stages. There was no significant difference in bud stage between treatment and treatment storage interaction but significant difference observed in storage condition. The significant level of moisture content (85.07%) was recorded by the treatment SNP 20ppm (T2) at bud stage when compared to control (T1) (80.26%). A similar trend of moisture content was noticed at bud opening and senescence stage which recorded 72.46 and 53.74% respectively by the same treatment and control (T1) registered the moisture content of 48.08 and 39.71% in bud opening and senescence stages under cold storage condition. Maximum moisture content was observed in the treatment NAA 100ppm (T5) with 72.58 per cent at bud stage, 38.76% at opening stage and 34.57 % at senescence stage while in control (T1) registered 65.91% (bud stage ), 32.82% (opening stage) and 26.43% ( senescence stage ) of moisture content at ambient storage condition.Significant difference recorded among different treatments and storage condition (Fig. 1). Rosa hybrida var Samantha kept in vase holding solution, after 4 days rapid decline moisture content was identified as the main cause for the flower senescence (Xue and Lin, 1999). Similar result, reduction in moisture content due to rapid water loss in petals has been observed in Rosa hybrida (Carpenter and Rasmussen, 1973) and Anthurium cv. Ozaki Red (Paull et al., 1985).
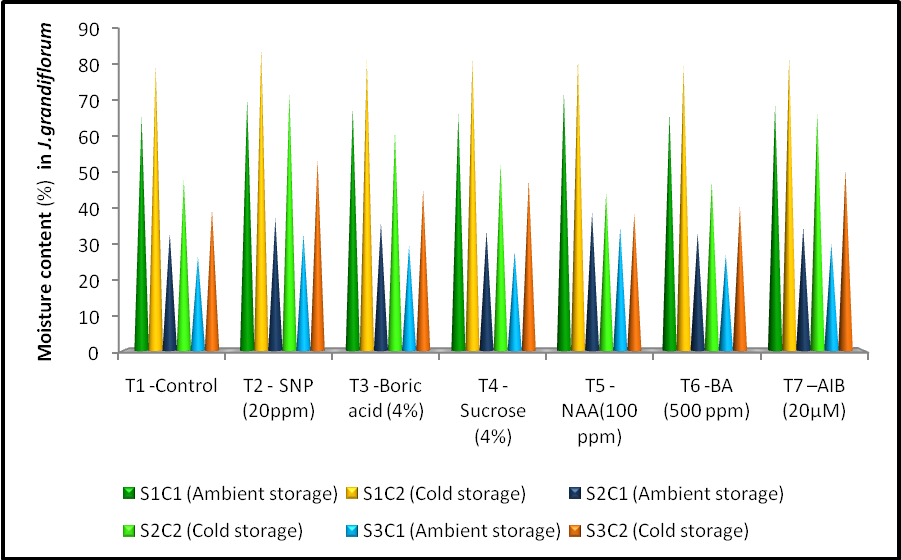 | Figure 1. Effect of different anti-senescence chemical treatment and storage condition on moisture content (%) at different stage in Jasminum grandiflorum |
3.2. Effect of Treatments and Storage Condition on Membrane Stability Index
- Among the chemical treatments and storage condition the treatment SNP 20ppm (T2) had the significant effect on Membrane Stability Index (MSI) of 99.46, 93.03, 74,12% at bud, opening and senescence stage compared with control (T1) which recorded minimum membrane stability index of 79.02,74.11, 65.54% at the same stages under cold storage condition.Treatments and storage conditions were significantly affect the MSI in J.grandiflorum flowers. 88.14, 78.69 and 68.13% is maximum membrane stability index observed in the treatment of NAA 100ppm (T5) compared with the control (T1) with 80.40, 52.87 and 50.04% at the respective stages of bud, opening and senescence stages under ambient storage condition.Membrane stability index getting decreased towards the senescence stage (Fig. 2). The MSI indicates the loss of membrane stability and cell death (Van Doorn, 2004) proceeding to the early petal senescence and decreased shelf life. In rose petals the loss of water content decreased with an ion leakage (Meeteren, 1979). Releases of the leachates into the interspaces of plant cells is a resultant process of disturbed water content leads to early breakdown of plasma membrane in cut flowers (Bhaskar et al., 2006). Sucrose is known to stabilize selective permeability of cell membrane (Santarius, 1973 and Van Doorn, 2004). Further, leakage of cell constituents due to loss of structural integrity of cell membrane results in death of flowers (Bhattacharjee, 2001).
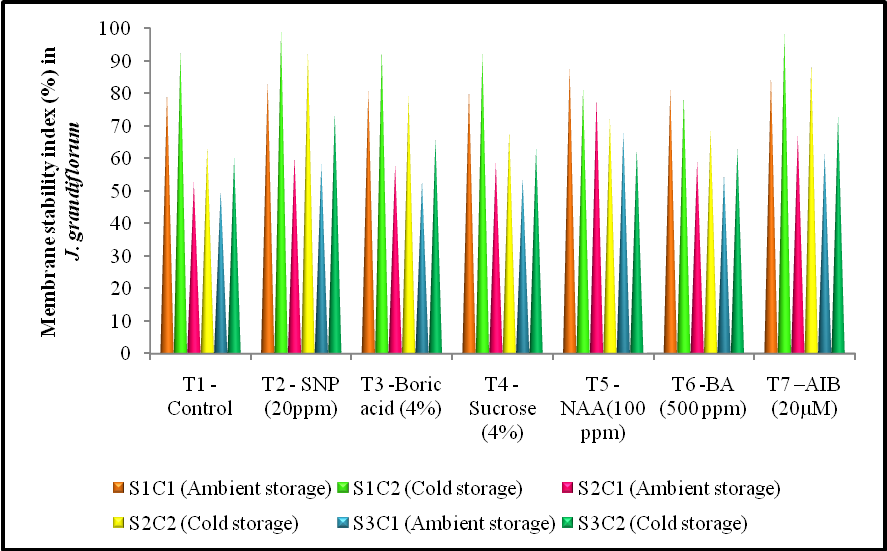 | Figure 2. Effect of different anti-senescence chemical treatment and storage condition on membrane stability index (%) at different stage in Jasminum grandiflorum |
3.3. Effect of Treatments and Storage Condition on Protein Content
- The data showed that anti-senescence chemical treatment and storage condition had significant effect on protein content of jasmine flowers at all the stages (Fig. 3). Maximum protein content was found in treatment of Silver Nano Particle 20 ppm (T2) with 52.78 mg g-1 over control (T1) in the value of 35.66 mg g-1 of protein content at bud stage in cold storage condition. The SNP 20 ppm recorded maximum protein content of 50.67 and 46.34 mg g-1 at opening and senescence stage respectively compared with control (T1) it recorded 31.3 and 23.42 mg g-1 of protein content at opening and senescence stage respectively.
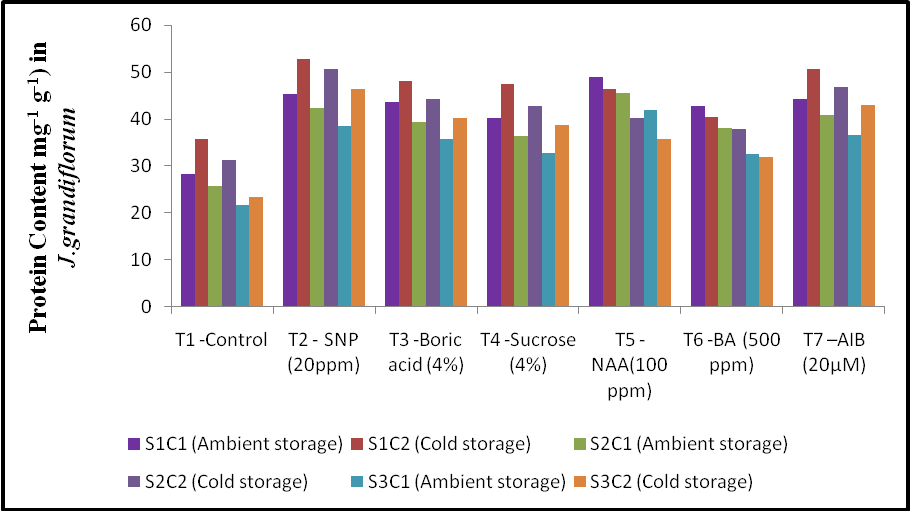 | Figure 3. Effect of different anti-senescence chemical treatment and storage condition on protein content (mg-1 g-1) at different stage in Jasminum grandiflorum |
3.4. Effect of Treatments and Storage Condition on Phenol Content
- The J.grandiflorum flower buds are treated with SNP 20 ppm (T2) registered significant total phenol content of 5.33 mg g-1 at bud stage and also shows significant low total phenol content in opening stage with 7.14 mg g-1 and 10.28 mg g-1 in senescence stage under cold storage condition. In case of control (T1) it registered maximum total phenol content compared with other treatments with 10.65 mg g-1 (bud stage), 12.56 mg g-1 (opening stage) and 14.77 mg g-1 (senescence stage) at cold storage condition. The data on total phenol content under ambient storage conditions was higher when compared to cold storage condition (Fig.4). All the treatment and storage condition shows significant effect on total phenol content. The least total phenol content were noticed in the treatment NAA 100ppm (T5) with 7.45 mg g-1 at bud stage, 8.07 mg g-1 at opening stage and 10.87 mg g-1 at senescence stage compared with control (T1) 12.19 mg g-1 at bud stage, 13.34 mg g-1 at opening stage and 15.87 mg g-1 at senescence stage in ambient storage condition.
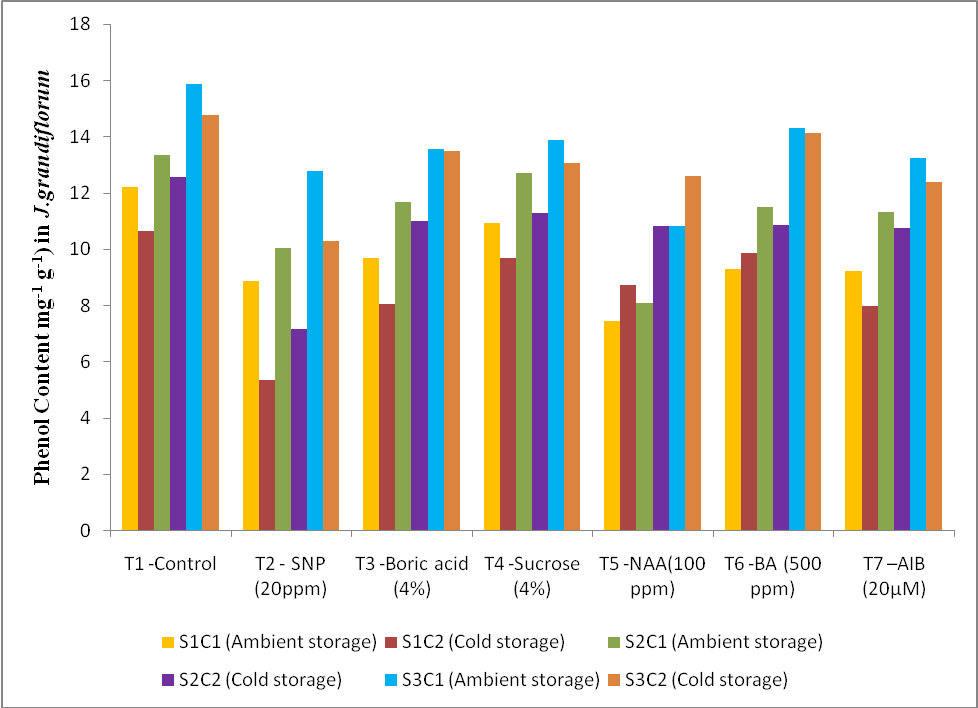 | Figure 4. Effect of different anti-senescence chemical treatment and storage condition on phenol content (mg-1 g-1) at different stage in Jasminum grandiflorum |
4. Conclusions
- This study concluded that flower buds treated with SNP 20 ppm under cold storage condition maintained the higher protein content, moisture content, membrane stability index with less phenol content which acts as the main key factors that maintain the structural integrity of cell and membrane of petals. By this way petals senescence delayed compared to other treatments and storage condition.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML