-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2018; 8(1): 32-38
doi:10.5923/j.als.20180801.03

A Role of Abscisic Acid in the Induction of Tuber Dormancy in Yam (Dioscorea alata L.)
Somina Braide, Elsie I. Hamadina
Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Dormancy prevents field yams tubers from sprouting, even when environmental conditions are favourable, until 270 days after tuber initiation. A possible role of abscisic acid (ABA) in this regard has been proposed but not established in yam. This study was conducted to assess the role of abscisic acid in the induction of tuber dormancy in D. alata tubers. Rooted minisetts were grown in a hydroponics system containing ABA, a competitive inhibitor of ABA (Fluridone), ABA followed by Fluridone, or non (the control; nutrient solution alone). The four treatments were replicated three times and arranged as Completely Randomized Design. Results show that Fluridone, and ABA + Fluridone treatments induced sprouting on new underground tubers as early as 34 DAT while the new underground tubers from the control, or ABA alone did not sprout even after 120 days in storage. Furthermore, tuber total carotenoid content followed the order Fluridone> control, Fluridone + ABA and ABA alone. Leaf stomata density and chlorophyll a and b content at 13 WAT (weeks after treatment) did not significantly account for the observed differences in number of sprouting underground tubers. In contrast, number of sprouting tubers was significantly (p< 0.05) influenced by leaf chlorophyll content at 7 WAT (not earlier) when measured as an index of greenness of leaf; y = -0.169x + 8.05 at p<0.05 R2=0.89. The number of sprouting tubers was significantly higher in treatment with whitish leaves (an effect of Fluridone). This study unequivocally shows that the ability of Fluridone to induce sprouting on tuber soon after formation relates to its whitening effect on leaves and to its effect on tuber caroteniod content both of which relate to the inhibition of ABA. Advancing this knowledge can lead to the development of a method(s) for inducing mid-season sprouting in field yam tubers and hence promote one than one season planting.
Keywords: Dioscorea alata, Dormancy, Abscisic Acid, Fluridone, Chlorophyll, Carotene
Cite this paper: Somina Braide, Elsie I. Hamadina, A Role of Abscisic Acid in the Induction of Tuber Dormancy in Yam (Dioscorea alata L.), Advances in Life Sciences, Vol. 8 No. 1, 2018, pp. 32-38. doi: 10.5923/j.als.20180801.03.
Article Outline
1. Introduction
- Yam (Dioscorea spp) is one of the oldest crops eaten in many parts of the world. It ranks second after cassava in West Africa as a major source of nutritional diet and income for the populace. Yams are grown in regions on three continents: Africa, Asia, and South America [1-3]. In West Africa, the most important species are white yam (D. rotundata) and water yam (D. alata) amongst others. It is a major source of nourishment to millions of people, as well as being a symbol of affluence. Culturally, yam plays a leading role in such activities as marriages and fertility, etc. [1, 4]. Also, the new yam festival celebrated in Nigeria sometime in August features yam tubers as the centre of all action. During the planting season, the tuber is again important for its role as the major source of planting material. Presently, yam is grown as an annual crop with its main planting period in February-April (in West Africa) and harvesting period I n August-September (first harvest) or November-December (second/main harvest). Yam production in both small and large-scale systems faces a major restraint - rarity of planting materials [5]. Efficient and effective methods to enhance the otherwise limited supply of yam tubers for planting must be sought. Adopted methods such as the storage of a fraction of harvested tubers, minisett technology and vine propagation. though not widespread have led to small increases in the availability of yam tubers for various purposes while in vitro microtuber propagation and the use of Temporary Immersion Bioreactors (TIB) for tuber production have helped with the production of clean tubers. Tubers that are produced via these methods or through the traditional method of propagation are unable to commence sprouting until about 4-6months after harvest depending on time of harvest (the earlier the harvest date, the longer the duration to sprouting), variety/species, environment, etc.This innate inability to sprout restricts the cultivation of yams to one season or cycle, slows down the rate of progress of crop development, and makes storage of seed tubers (whole ware or small tubers or tuber portions reserved for planting) compulsory even if conditions for growth were available. These in turn impose extra costs; for provision of storage facility, exposes stored tubers to many undesirable conditions such as: unplanned consumption, pests and diseases attack, and leads to reduction in the quantity of tubers available for planting in the next season [6, 7]. Developing methodsthat induce early sprouting on soil grown seed tubers is, therefore, an option for reversing the consequences outlined above.The inability of yam tubers to sprout earlier than the defined planting/sprouting time is controlled by the phenomenon called Dormancy. Dormancy is an adaptive mechanism that imposes a programmed inability on materials, however viable, to grow owing to factors that are endogenous or exogenous to the tubers. According to [8], three phases of dormancy are proposed; Phase 1 commences with tuber initiation to the appearance of tuber germinating meristem (TGM), Phase 2 is characterized by the appearance of tuber germinating meristem to the initiation of foliar primordium and Phase 3 begins from the initiation of foliar primordium to the appearance of the shoot bud on the surface of the tuber. Phase 1 is regarded as the longest phase of dormancy, lasting about 200 days and up to 220 days in TDr 131. This phase of dormancy is proposed to be under the control of factors endogenous to the tuber (endo-dormant phase). Phase 2 and 3 together are shorter (<70 days) than Phase1. They are influenced by PGRs and environmental conditions and are thus, referred to as endo/eco-dormant phases. Undoubtedly, a promising advance to solving the problem of long tuber dormancy is one that is aimed at preventing the initiation of dormancy or shortening the long Phase 1 rather than to shorten the duration of the short Phase 2-3 of dormancy [8].In vitro studies have shown that yam plantlets derived from meristem culture can be induced to commence sprouting at or soon after tuber initiation by growing them in culture media containing the agrochemical - Fluridone at varying concentrations [9, 10]. To determine whether Fluridone might induce sprouting on seed yams produced from field grown yam tubers, [11] designed a hydroponics system that allow plant roots to absorb Fluridone during the early stages of the Phase 1 of tuber dormancy. Their study showed that Fluridone can cause bleaching of leaves and lead to precocious sprouting of seed tubers. The outcome of these Fluridone studies were indicative of a strong participation of the plant hormone abscisic acid (ABA) in the induction and maintenance of yam tuber dormancy. This is because Fluridone is famed for its ability to inhibit ABA biosynthesis by blocking a important step in the pathway of ABA formation, ie., the conversion of phytoene, a C40 caroteniod and precursor pigment for the formation of ABA, to carotene [12, 13]. Nonetheless, the yam fluridone studies do not unequivocally prove the involvement of ABA in yam dormancy since the studies did not include an ABA treatment and an ABA reversal treatment (Fluridone+ABA). Therefore, this study seeks to answer the following questions: would the uptake of Fluridone by non-tuberizing plants derived from minisetts cause precocious sprouting on the new tubers (i.e., seed tubers) that will be formed? Is ABA involved in the induction of dormancy in D. alata? What is the effect of Fluridone on leaf chlorophyll, tuber and leaf carotene content and stomata density? Hence the objectives of this study are: (1) to determine the effect of 30µM Fluridone and ABA on the timing of sprouting of seed tubers, and (2) to determine the effect of 30µM Fluridone on leaf and tuber carotene content.
2. Materials and Method
2.1. Study Environment
- This study was a screen-house study conducted in the Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt. The top ¼ of the screen-house was constructed with plastic mesh sheet while the rest was covered with transparent polyethylene sheet. The structure was roofed with plastic roofing sheets. The photosynthetic active radiation (PAR) level in and outside the screen-house was monitored using a quantum PAR meter (Hydro Farm product, USA). This was necessary since the rate of photosynthesis in plants is related to the number of photons absorbed in the 400 to 700 nm band of the electromagnetic spectrum. Temperature and relative humidity in and outside the screen-house were also monitored.
2.2. Planting Material
- Tubers of Dioscorea alata var TDa 98/01166 were used in this study. Tubers of this species were collected in the year 2015 from National Root Crops Research Institute, Umudike, and multiplied over two seasons. The tubers used in this study were harvested in December 2016. TDa 98/01166 was chosen for this study because it is known to exhibit long tuber dormancy and presents a potential commercial value as an alternative source of non-dietary and dietary requirements. Ability to shorten dormancy and hence produce more than one crop per season would relieve some pressure on the more accepted variety, D. rotundata.
2.3. Preparation of Minisetts
- Minisetts were obtained from the proximal region of the non-dormant tubers. The tuber-head, which is the corm-like structure attached to the proximal region of the tuber [7, 13] was severed from the tuber (where present) to inhibit apical dominance. Minisetts weighing approximately 50 g were obtained by longitudinally cutting through them making sure that every minisett had a measure of the head region because the head region of tubers are known to have greater tendency to sprout than the middle or distal regions [15]. The cut ends of all the minisetts were treated by dabbing them in wood ash to minimize rotting [16].Treated minisetts were air-dried for 24h then pre-sprouted in baskets filled with moist sawdust. The base and sides of the baskets were lined with 2mm net to prevent loss of the sawdust through the holes. Daily observation of the minisetts for vine emergence was done. Watering was done when necessary. At 10 days after pre-sprouting, most of the minisetts had initiated sprouts.
2.4. Rooting of Pre-Sprouted Minisetts
- Pre-sprouted minisetts were made to root in polybags containing soil and well cured poultry manure mix in the ratio 25:1. Watering was done when necessary.
2.5. Transplanting of Rooted Plants to Hydroponics Systems
- Rooted plants were transferred to a hydroponics system on 14 July 2017. Soil on individual plants was shaken off to expose the roots and then washed before transplanting them randomly into 5 inches long plastic pots containing 100 ml distilled water. The pots were perforated in sets of threes along the side of the pots with the first perforation done 1 inch above the base. The pots were disinfected and thoroughly rinsed before use. Disinfected (in 5% sodium hypochlorite) coco coir was used to support for the plants and to keep the new tubers (when they are initiated) away from light while the roots were placed at the base of the pot to enable water/treatment absorption.
2.6. Solutions and Reagents
- Standard Hoagland nutrient solution was used for the hydroponics treatments throughout the study. Fluridone and ABA were purchased from ChemServices, USA.
2.7. Experimental Treatments and Application
- Treatments were applied two days after plants were transferred to the hydroponics system. All test compounds were added to the basic nutrient solution. The experiment consisted of four treatments: Nutrient solution (control), 30 µM Fluridone, 150 µM ABA, 30 µM Fluridone +150 µM ABA applied one week later. Fluridone and ABA were chosen based on existing knowledge of their effects on yam dormancy [8, 11]. 30 instead of 10 µM Fluridone was chosen because bigger plants are expected with the planting of 50g mini-setts. All treatments were applied at a rate of 100 ml per plant. Treatments were applied every two days until three cycles were achieved. In the Fluridone+ ABA treatment, two cycles of 150 µM ABA were applied at the end of the three cycles of 30 µM Fluridone.
2.8. Experimental Design and Data Collection
- The experiment was designed as a Completely Randomised Design (CRD) with four treatments replicated three times with one plant per pot and six pots per treatment per replicate.Sprouting date, Number of sprouts per tuber and number of tubers per sprouting plant: All plants were observed for the above. The date of appearance of sprout(s) on newly initiated tuber(s) was recorded. Number of sprouts present, and number of tubers per sprouting plant was counted at 3, 5 and 7 WAT, and the data was used to determine the number of sprouts produced and number of sprouts per tuber. The date of sprout appearance and number of sprouts produced were used to determine emergence percentage (EP), emergence speed index (ESI) and mean time to emergence (MTE). In situ leaf chlorophyll content: In situ leaf chlorophyll content was determined at 4, 5 and 7 WAT using a hand-held chlorophyll meter, atLEAF CHL STD meter. The chlorophyll meter compares the transmission of light in red and near infrared wavelengths to give a measure of chlorophyll content in leaves. A chlorophyll value above 35 suggests that the plant is healthy. Two readings per leaf were taken on four representative leaves per plant and two plants per treatment per replicate. Tuber and leaf carotene contents, and chlorophyll a and b content: At the end of the experiment (13 WAT), two plants/treatment/replicate were sampled and twelve green leaves were taken from each of the plants for the analyses of chlorophyll a and b, and leaf carotene following methods by [17]. Quadruplicate 1.0 g leaf tissue samples were analysed. Pigment measurements were quantified spectrophotometrically using a GENESYSTM 10 Series Spectrophotometer by Thermo Electron Corporation. Absorbances of chlorophylls a and b and carotenoids (x + c) extracts were determined at wavelengths of 662, 645 and 470 nm, respectively; where (x + c)= Total carotenoids, xanthophylls and carotenes. To calculate concentration (µg ml-1 FW) of pigments, [18] equations were used. The whitish leaves on Fluridone treated plants were not used for the analysis. The new tubers produced by the sampled plants were used for the determination of tuber carotene content following methods by [17].Determination of dry matter content: At the end of the experiment (13 WAT), two plants per treatment/rep were sampled for this analysis. They were separated into their component parts, dried in a forced air oven at 70°C. Number of tubers per plant was recorded.Growth measurements: Growth data was collection at 2, 3, 4, and 5 WAT on two pre tagged plants per treatment per rep. Leaf length and width were repeatedly measured on six leaves per plant. Number of leaves per plant was counted. Stomata count: At the end of the experiment (at 13 WAT), three leaves/treatment /rep were randomly selected for determination of stomata density. The stomata count was done at two Fields of View per leaf and observations were done at 100x using a light microscope.Dormancy of non-sprouting tubers: At the end of the experiment, non-sprouting underground tubers from two plants per treatment per replicate were weighed, placed in baskets and stored under ambient conditions for three months. Tubers were observed for date of sprouting. Data was used to determine duration of dormancy, at least to the end of the study.
2.9. Data Analysis
- Emergence Percentage (EP): Emergence percentage was calculated using the equation below:
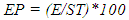 | (1) |
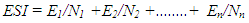 | (2) |
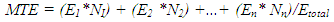 | (3) |
3. Results and Discussion
3.1. Growing Conditions
- The average temperature and relative humidity inside and outside the screen-house varied only slightly. The average temperature at 8am, 2pm and 6pm in the screenhouse was 22.8, 29.2, and 26.5°C respectively while average relative humidity at the same times were 82.7, 76.3 and 82.6% respectively. The average photosynthetic active radiation (PAR) reading at lower and upper plant levels was 88 and 124 µmoles/m2/s respectively.
3.2. Effect of Treatments on Morphology
- Plants treated with Fluridone expressed observable colour change at four weeks after treatment (Fig. 1ab). Some of the leaves showed extensive bleaching with a purplish and/or white stem/vine. Bleaching was complete in some leaves and patchy in others. The leaves of plants growing in nutrient solution plus ABA alone and nutrient solution alone (control) expressed no bleaching of leaves or other plant parts. Treatments had no effect on position of tuber formation and/or shape of tubers.
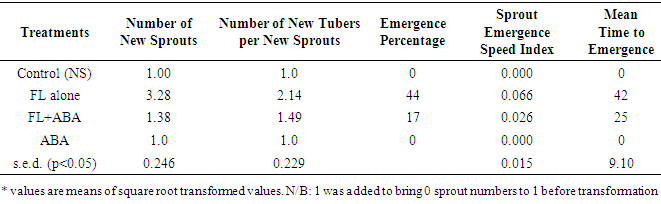
3.3. Effect of Treatment on Sprouting
- Number of new sprouts, number of new tubers per sprout and time to sprouting: Sprouting, which is a strong indicator of the absence of dormancy, was first observed (above the surface of the cococoir), at 34 d after treatment (i.e. 5 WAT) but only on the new underground tubers that developed in Fluridone treatments. By the end of the study (13 WAT), up to 44% of Fluridone treated plants produced sprouting tubers and this declined systematically to 17% in Fl+ABA and then 0% in both ABA and the control (Table 1). The number of new sprouts per new tuber and the number of new tubers per sprout varied significantly (p<0.05) indicating that the Fluridone treatments were more likely to produce more than one sprout per tuber as well as more than one tuber per sprout. The number of new sprouts per tuber ranged from 1 to 8 under Fluridone and the number declined significantly with the inclusion of ABA in the solution (FL+ABA). There was zero number of sprouts in both the control and ABA treatments.The emergence speed index (ESI) showed that more tubers sprouted per week under Fluridone and that the addition of ABA one week after Fluridone caused a significant decline in ESI with the ESI value declining by more than half (Table 1). Also, there were no significant differences in the number of new sprouts on aerial tubers across the Fluridone treatments. Fig. 2a and 2b. shows the new sprouts from aerial and underground tubers.
|
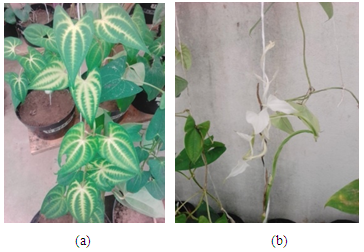 | Figure 1. a) Patchy Bleached and b) Bleached Leaves in Fluridone Treatments |
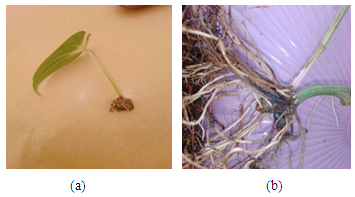 | Figure 2. Sprouting Aerial (a), and Sprouting Underground (b) New Tubers |
3.4. Effect of Treatment on Vegetative Growth
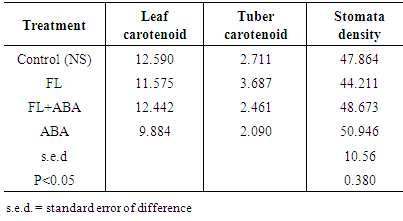
- Leaf length and width, and leaf number: Leaf length and width did not vary significantly across treatments throughout the study. The mean leaf length (square root transformed) in the control at 2, 3, 4 and 5 weeks after treatment was 9.3 and 9.9, 9.2 9.3 respectively. The average leaf weight, in the control, at 2, 3, 4 and 5 weeks after treatment, was 7.1, 7.2, 7.3, 7.4 respectively. Similarly, there was no significant difference in the mean number of leaves (square root transformed) across treatments at 2, 3, 4 and 5 WAT. The average number of leaves, in the control, at 2, 3, 4 and 5 weeks after treatment was 4.6, 4.9, 5.8 and 6.2 respectively. Dry matter content: Data on dry matter content at 8 WAT showed that stem (petiole inclusive), leaves, root and tuber dry matter content did not vary significantly (p<0.05) across treatments. However, the trend showed that weights declined slightly in Fluridone treatments compared to the control while ABA alone increased the dry weights compared to Fluridone treatments. Dry weights of the various parts were slightly higher under ABA alone than the control. Number of tubers produced: Analysis of the number of tubers produced per treatment at the end of the study (at 8 WAT) shows that plants produced 1 to 8 underground tubers per plant. Although there was no significant difference across treatments in number of tubers, a trend was noted; ABA alone produced the highest number of tubers (1.496 sqr transformation) > FL + ABA (1.275) > FL alone (1.138) > Nutrient solution alone (1.039) (control). Thus, it appears that ABA tends to increase tuber number compared to Fluridone while Fluridone tends to increase tuber number compared to the control.Chlorophyll content: In situ leaf chlorophyll data (using the atLeaf handheld chlorophyll meter) showed that leaf chlorophyll content varied significantly across treatments at 4 and 7 WAT (Table 2). The atLeaf handheld chlorophyll meter compares the transmission of light in red and near infrared wavelengths to give a measure of chlorophyll content in green leaves. Chlorophyll levels were within acceptable range in most treatments; mean chlorophyll values were mostly above the critical value of 35. At 5WAT, Fluridone treatments did not significantly affect chlorophyll content while ABA significantly increased chlorophyll content compared to the control or Fluridone treatments.
|
|
4. Conclusions
- This study has shown that the ability of Fluridone to induce sprouting on yam tubers relate to its ability to cause whitening of leaves and increase tuber carotenoid content both of which relates to ABA inhibition. Also, this study has shown that, the absorption of Fluridone by a yam plant; derived from 50g portions of ware yam tuber, prior to its new tuber formation leads to sprouting of the new tuber/seed tuber soon after it is initiated. These findings have contributed to the understanding of the role control of yam tuber dormancy and will be helpful in the development of cheaper, less skill requiring method(s) of inducing sprouting in dormant yam tubers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML