-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2017; 7(2): 15-20
doi:10.5923/j.als.20170702.01

Phytotelmata Might Account for the High Prevalence of Dengue Hemorrhagic Fever in Lampung, Indonesia
Emantis Rosa, Mohammad Kanedi, Putri Minggar Okatviani, Welmi Nopia Ningsih
Departmen of Biology, Faculty of Mathematics and Sciences, Lampung University, Indonesia
Correspondence to: Mohammad Kanedi, Departmen of Biology, Faculty of Mathematics and Sciences, Lampung University, Indonesia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It was revealed that phytotelmata is an importance breeding place for numerous types of insect including the vector mosquitoes of deadly diseases such as dengue and malaria. Yet, such small ponds tend to be neglected by the country health authorities in setting eradication program of the disease vectors. This study aimed to find out whether phytotelmata contribute in providing breeding place for vector mosquito of dengue hemorrhagic fever (DHF)- the Ades larvae. The survey carried out in four districts namely Pesawaran, Metro, Pringsewu and Bandar Lampung and lasted from March to August 2016. Phytotelmic criteria of the study were every structures present in plants which allow rain water to impound and showed the signs of life exist in it. The phytotelmata containing Aedes mosquito larvae were assessed for its physical parameters and the data resulted were analysed and presented descriptively. There were six types of phytotelmata found namely leaf axils, tree holes, tree stumps, fallen spathe, fruit shells, and fallen leaves. The Aedes larvae found belong to only two species, i.e. Aedes albopictus(n=373) and Aedes crysolineatus (n=26).
Keywords: Phytotelmata, Mosquito breeding place, Dengue fever, Aedes larvae, Vector mosquito
Cite this paper: Emantis Rosa, Mohammad Kanedi, Putri Minggar Okatviani, Welmi Nopia Ningsih, Phytotelmata Might Account for the High Prevalence of Dengue Hemorrhagic Fever in Lampung, Indonesia, Advances in Life Sciences, Vol. 7 No. 2, 2017, pp. 15-20. doi: 10.5923/j.als.20170702.01.
1. Introduction
- Dengue hemorrhagic fever (DHF) is one of the mosquito-borne diseases that is prevalent in the Indonesian provinces, including Lampung. It said prevalent due to the occurrence of the disease is seasonal in pattern and often be an outbreak in a certain area. In the period of 2000-2012, in the province of Lampung with a population of about 8 million, more than 14,174 people suffered from DHF and 151 of them died [1, 2]. Despite the government, both national and local, have been implementing a variety of strategies to eradicate the diseases, among others, through the eradication of the vector but in fact, this disease still occur annually. The question, then, why is it so hard to prevent the disease, especially in relation to vector control? As is known, most Indonesian people have been briefed by the authorities for vector control programs against deadly diseases such as dengue and malaria through the insertion of these materials into primary and secondary school curricula. In the context of DHF, one of the most information known to the community is that this disease is transmitted by the mosquito Aedes aegypti [3]. One of the shortcomings of vector control programs in use today, particularly against mosquitoes, is ignoring that the breeding places of the vectors are not just limited to the ponds, water container, or a variety of man-made waste items such as discarded tyres or used cans. In fact, various species of vector mosquitoes such as Aedes sp. were found to breed in a very small water bodies trapped in tree holes, leaf axils, bamboo joints, or papaya stumps [4, 5]. Such small water bodies found in or upon plants are called, by Ludwig Varga in 1928, as phytotelmata [6]. Unfortunately, in Indonesia, study on phytotelmata has not been done in earnest [7]. As a result, the importance of phytotelmata in the epidemiological fields and health management programs has not been put into consideration.This study aimed to find out whether phytotelmata contribute in providing breeding place for mosquito vector of DHF in Lampung, an Indonesian province situated in the most southern part of Sumatra, by identifying mosquito larvae of Aedes inhabit the phytotelmata.
2. Method
- Study Area The study was carried out from the end of rainy season until the peak of dry season, March –July 2016. The survey was targeting four districts in Lampung Province namely Pesawaran, Metro, Pringsewu and Bandar Lampung. Pesawaran, is known as the district with the highest malaria endemicity in which one of the sub-district ever records the levels of annual parasite incidence (API) up to 51.8. Furthermore, Metro, Bandar Lampung and Pringsewu are the three districts that were ranked first, second and third highest for the DHF incident rate in 2012 [8]. Inclusion Criteria The phytotelmic criteria of the study was adopted from Kicthing [6] i.e. every structures present in terrestrial plants such as modified leaves, leaf axils, flowers, stem holes or depressions, tree stumps, open fruits and fallen leaves, which allow rain water to impound and in the impounded water the signs of life was seen visually. Plants that were observed include ornamental plants grown in backyard, wild plants and crop plants that grown naturally or cultivated in plantation areas.Sampling Each plant or its parts found to contained phytotelmata was photographed or sampled for further taxonomic identification. The impounded water in each phytotelmata was collected using a hose connected to a manual suction pump, but before being discharged the phytotelmic water temperature was measured. The phytotelmic fluid then placed separately in labeled vials and transported to the laboratory for further analysis. At the laboratory, the mosquito larvae were sorted and separated from trash and other debris. Then, the volume and pH of the phytotelmic water were assessed. The dead larvae were separated from the living larvae and put into bottles containing 70% alcohol for toxonomic identifiaction. Furthermore, the living larvae were kept alive and reared until reachs mature stage. Both the dead and living larvae of the mosquitoes were examined under a stereo microscope Nikon Olympus SZ51. The taxonomic procedures for larval identification and determination of genus Aedes referring to the work of Farajollahi and Price [9]. Data AnalysisThe data resulted from the study were presented and analysed using descriptive statistics.
3. Results
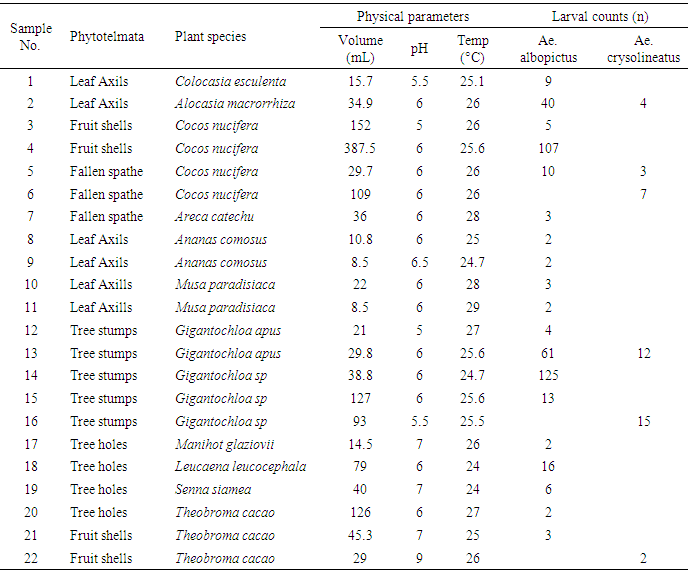
- There were five types phytotelmata found in the study namely leaf axils, tree holes, fallen spathe, fallen leaves, and open fruit shells (Fig. 1). Plant species the complete list of plant species with parts that fulfil the inclusion criteria as phytotelmata found in the study are presented in Table 1. Of all the phytotelmic sample, 22 of them were found to contain larvae of Aedes mosquito. The larval count of the Aedes mosquitoes and the physical parameters of the phytotelmic water are presented in Table 2.The data in Table 2 can be elaborated as follows. There are two species of Aedes mosquito larvae managed to be identified in this study, i.e. Ae.albopictus and Ae.crysolineatus. The larvae can be found in the phytotelmata with a volume as low as of 8.5 ml, i.e. in the leaf axils of pineapple (Ananas comosus) and banana (Musa paradisiaca). In average, a single larvae of Ae. albopictus can even live in the water column with a volume of only 0.31 ml, such as in the hole of bamboo stumps where 125 larvae inhabit water column with a volume of 38.8 ml. Phytotelmic water temperature that can be occupied by Aedes larvae is ranged from 24 to 29°C, but seemingly the most preferred temperature is 24-26°C (Fig. 2). Phytotelmic water pH range where Aedes larvae are found is 5.5 - 9, with the most preferred pH by both species is 6 (Fig. 3).
 | Figure 1. Samples of plant parts that impound water serve as phytotelmata found in Lampung |
|
|
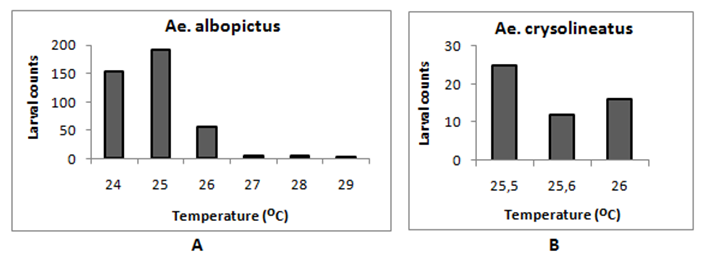 | Figure 2. Range of phytotelmic water temperature that are occupied and preferred by Ae albopictus (A) and Ae crysolineatus (B) |
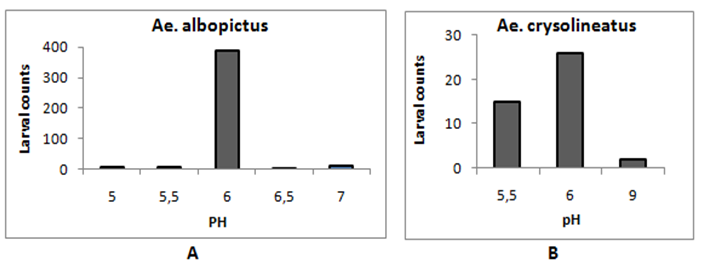 | Figure 3. Range of phytotelmic water pH found inhabited and preferred by Ae albopictus (A) and Ae crysolineatus (B) |
4. Discussion
- This research was conducted in 2016, the year in which 4.523 people in the studied province found suffered from DHF and 15 of them were died. In the surveyed district namely Pesawaran, Metro, Pringsewu and Bandar Lampung, the DHF patients consecutively were 324 (one died), 222 (two died), 684 (three died) and 826 (one died) [10]. This study has confirmed the findings of previous studies that in nature, mosquito larvae, including the larvae of Culex spp. [11], Ochlerotatus notoscriptus [12] and Ae albopictus can breed in various small water bodies including the dried fallen leaves and tree holes [13]. Interestingly, besides Ae. albopictus and Ae. crysolineatus, this study did not find any Ae. aegypti mosquito larvae in each phytotelmata that was investigated. The question is why?The first possibility is Ae aegypti mosquito prefers to breed indoor in a small water body such as in the open masonry tanks used for water storage, or out door in a small water body trapped in the discarded goods such as old tyres and cans [14, 15]. The second possibility is the DHF in the area surveyed is not only transmitted by the Ae aegypti mosquitoes but also, or mostly, by the Ae albopictus. It is very likely for the Ae albopictus, commonly called as the Asian tiger mosquito, is known as a secondary vector of dengue [16]. In Indonesia alone, precisely in Banjarnegara, Central Java, the role of Ae albopictus in dengue disease transmission is indicated by Rahayu and Ustiawan [17]. Of course, it should be recognized that the morphological appearance of both species is very resemble to each other. A strong evidence that Ae albopictus can contribute to the spread of dengue demonstrated by Rosa et al. [18]. By using RT-PCR methods for detecting dengue viruses on Ae.albopictus larvae inhabit phytotelmata they found two serotypes of dengue viruses DEN-1 and DEN-4 and suggested that the mosquito has the potential to transmit and spread DHF.The third possibility which account for Ae.aegypti are not found in phytotelmata is the larvae allegedly did not manage to compete with Ae. albopictus larvae. As shown by a study carried out in Malaysia, Ae.aegypti has lower competitive advantage compared to Ae.albopictus in situation of limited resources. This situation allegedly the cause of the dominancy of Ae. albopictus mosquito in Penang Island [19]. The high adaptability of Ae albopictus is illustrated by the extent of the spread of mosquitoes that originally indigenous to South-east Asia and islands of the Western Pacific and Indian Ocean, to Africa, the mid-east Europe and the Americas (north and south) [20, 21]. Lastly, the conditions that may lead to the absence of Ae aegypti in the phytotelmata samples found in this study are the difference of physical condition suitable for the larvae. As revealed from an experimental study, the best water temperature for the Ae aegypti mosquito larvae to grow is 30°C [22], while the temperature of the phytotelmic water in where the larvae of Ae albopictus found in this study is 24 - 29°C.
5. Conclusions
- This research was conducted in the year in which the incidence rate of DHF was high, however the study did not manage to find Ae aegypti in addition to Ae albopictus and Ae crysolineatus. Given Ae albopictus was revealed to contain viruses DEN 1 and DEN 4, and has been long known as a secondary vector of dengue fever, then it can be assumed that Ae albopictus could potentially be the DHF vector and phytotelmata might account for the high prevalence of the disease in Lampung, Indonesia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML