-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2016; 6(3): 54-60
doi:10.5923/j.als.20160603.02

Interspecies Differences on Ovarian Parameters between Black Bengal Goat and Indigenous Bengal Sheep in View of In vitro Maturation
S. A. Masudul Hoque1, Md. Mazharul Islam1, Abu Sadeque Md. Selim2
1Department of Animal Breeding & Genetics, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
2Department of Animal Science & Nutrition, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
Correspondence to: S. A. Masudul Hoque, Department of Animal Breeding & Genetics, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Black Bengal goat and indigenous Bengal sheep are the most important small ruminants in Bangladesh. To characterize the ovarian parameters and to study interspecies variation this research was conducted. Ovaries were collected from local slaughterhouse and categorized as left/right and CL-present/absent. After evaluation in terms of weight, length, width, height, number of atretic and non-atretic follicle in; Cumulus Oocyte Complexes were collected from 90% of the ovaries and remaining 10% were subjected to histological study. After tissue preparation and staining with Haematoxylene and Eosin stain, the diameter of primordial, primary and secondary follicles; thickness of follicular layers of graffian follicles were measured. It was observed that left and right ovaries were equally active to normal physiological and/or ovarian activity, as the parameters of both ovaries in both species were similar (P>0.05). The weight, length, width and height of CL present ovaries were significantly lower (P<0.05) but the number of non-atretic follicles, number of normal and total COCs were significantly higher (P<0.05) than CL present type in both species. The results of species comparison showed that all the parameters in sheep were significantly higher (P<0.05) than those of goat. In histological study, it was observed that the diameter of primary and secondary follicle, thickness of theca interna and theca externa in graffian follicle in goat were significantly higher than those of sheep.
Keywords: Primary, Secondary and Graffian follicles, Cumulus oocyte complexes (COCs), Black Bengal goat, Indigenous Bengal sheep
Cite this paper: S. A. Masudul Hoque, Md. Mazharul Islam, Abu Sadeque Md. Selim, Interspecies Differences on Ovarian Parameters between Black Bengal Goat and Indigenous Bengal Sheep in View of In vitro Maturation, Advances in Life Sciences, Vol. 6 No. 3, 2016, pp. 54-60. doi: 10.5923/j.als.20160603.02.
Article Outline
1. Introduction
- Bangladesh is an agricultural based country and livestock plays a vital role in national economy. Among the livestock species there are about 14.8 million goats and 1.9 million sheep in Bangladesh. Goat and sheep play a major role in modern agriculture and their size and physiology provide an appropriate model to study a variety of mammalian biological functions, including reproduction, embryology and fetal development. They have several heritable traits of economic interest and possess a large genetic variation between species and between breeds. These genetic variations occur in different breeds and provide interesting models to study the mechanisms regulating the formation and development of ovarian follicles. The latest developments in gametes and embryo cellular biology, the field of molecular embryology of farm animals has been poorly explored and genetic improvement of farm animals could be made by planned Artificial Insemination (AI) with frozen semen and Estrus Synchronization (ES) [1]. After a dramatic development of cellular biology over the last ten-fifteen years, many research efforts have been moved towards the implementation of embryo technologies involving Multiple Ovulation and Embryo Transfer (MOET), In Vitro Production (IVP) of embryos, Cloning and Transgenesis to transfer a targeted number of embryos having desired genetic make-up [2]. Considering the poor ovulatory response in small animal the application of MOET is difficult to adopt [3]. Nevertheless, it has not become widely used in goat and sheep due to its unpredictability [4]. Besides this, animal welfare concerns have more and more limitation on the use of animals for experimentation. For these reasons, IVP of embryos has received great attention and support in recent years [5, 6]. The IVP of embryos usually includes retrieval of oocytes from the ovarian follicles of a female, in vitro maturation (IVM), in vitro fertilization (IVF) of oocytes and in vitro culture (IVC) of presumptive zygotes to the morula or blastocyst stage [7]. The IVM is the primary process of IVP of embryos. It is well established that IVM of oocyte is divided into nuclear and cytoplasmic processes. Nuclear maturation involves resumption of meiosis and progression to metaphase-II stage. Cytoplasmic maturation encompasses a variety of cellular processes that must be completed for the oocytes to be fertilized and developed into a normal embryo and offspring [8]. The IVM is very much dependent on the quality of ovary, ovarian follicles and oocytes [1, 2, 9, 10]. In mammalian species, follicles begin to grow from a poo1 of primordial follicles constituted early in life, continuously throughout the life of the female. Primordial follicles are the least developed and most numerous follicles of the ovary. Each consists of a primary oocytes surrounded by a rarer of simple squamous follicular cells. Later on it developed to primary, secondary and graffian follicles, respectively [11]. In abroad, quantitative and histological aspects of ovary and ovarian follicles have been studied in sheep [12], bovine [13], mouse [14], wapiti [15] and Iranian Lori-Bakhtian Sheep and native Goat [16]. However, until today, there is no complete study on qualitative, quantitative and histological analysis on the ovary, ovarian follicle and oocytes in black Bengal goat or indigenous Bengal sheep. Keeping the aforesaid reality in mind the present research work has been undertaken with the following objectives- (i) to obtain complete information on the qualitative, quantitative and histological parameters of Black Bengal goat and Indigenous Bengal sheep in view of IVM of oocytes (ii) to compare the parameters between two species and (iii) to understand the pattern of follicle development in both species.
2. Materials and Methods
2.1. Collection, Processing and Evaluation of Ovaries
- Ovaries from goats and sheep of unknown reproductive history were collected from local slaughter house. The ovaries were kept in collection vial containing 0.9% physiological saline added with zentamycin (1000 mg per liter of saline solution) in a thermo flask at 250C to 300C and transported to the laboratory within 4 to 5 hours of slaughter. The ovaries were rinsed thoroughly and recorded as right and left ovary. The presence or absence of corpus luteum (CL) in both sided ovary was also recorded. In the laboratory, each ovary was trimmed to remove the surrounding tissues and overlying bursa. Each ovary was treated to three washings in D-PBS. Immediately after trimming, the weight (gm) of each ovary was measured by a digital electric balance and the dimensions (length and width in cm) were measured using a slide calliper.
2.2. Evaluation of the Ovarian Follicles
- After evaluation of each ovary, the number of visible follicles was counted and then follicular health was assessed according to Kruip and Dieleman [17] as- (a) Non-atretic: uniform bright appearance, extensive and very fine vascularization and no free-floating particles in the follicular fluid. (b) Atretic: loss of translucency, slightly or dull grayish and/or opaque appearance and free-floating particles in follicular fluid. Thereafter, 90% of the ovaries at each collection were subjected to oocyte collection and remaining 10% were stored in 10% formalin solution for Histological study.
2.3. Collection and Evaluation of COCs
- After necessary washing COCs were harvested by aspiration technique. For this, a 10 ml syringe was loaded with PBS (1-1.5ml), and the needle (19G) was put in the ovary parenchyma near the vesicular follicles and aspirated near the point at the same time. After aspirating the follicles from one ovary, the aspirated follicular materials were transferred slowly into a 90-mm petridish, avoiding damage to the cumulus cells. The petridishes were kept undisturbed for 5 min, allowing the oocytes to settle down. Excess media were taken out by a syringe without disturbing the oocytes at the bottom of petridish. The oocytes were then examined under a compound microscope at 10x magnification, and the total number of oocytes harvested was counted. The oocytes were classified into 4 grades on the basis of cumulus cells and nucleus as described by Khandoker et. al. [18], briefly; Grade A: Oocytes completely surrounded by cumulus cells; Grade B: Oocytes partially surrounded by cumulus cells; Grade C: Oocytes not surrounded by cumulus cells and Grade D: Degeneration observed in both oocytes and cumulus cells. The grade A and B were considered as normal and grade C and D as abnormal COCs.
2.4. Histological Study
- Histological slides from both left and right ovaries were prepared through fixation with 10% formalin solution, washing with running water, dehydration with alcohol, clearing with xylene, embedding in paraffin, sectioning in microtome and finally staining with Haematoxylene and Eosin according to Kabiraj et. al. [19]. Then, under microscope the ovarian follicles were recognized and classified into (i) Primordial follicle, (ii) Primary follicle (single layer, multi-layer), (iii) Secondary follicle with C shaped antrum and (iv) Graffian follicle. Then, diameter of follicles, thickness of follicular layers (granulosa, theca interns and theca externa) by micrometry method was measured as suggested by Mohanunadpour [16].
2.5. Statistical Analysis
- The data generated from this experiment were entered in Microsoft Excel worksheet, organized and processed for further analysis. Analysis was performed with the help of Statistical Analysis System [27]. The relationships among different parameters and microscopic structures obtained from the histological study were compared between Black Bengal goat and Indigenous sheep.
3. Results and Discussion
- Ovaries of Black Bengal goat and Indigenous Bengal sheep were collected from local slaughterhouses and recorded as right and left. After trimming and necessary preparation these were further categorized as corpus luteum (CL)-present or CL-absent group. In case of goat, among 386 ovaries CL was found in 162 ovaries with the remaining 224 ovaries having no CL. In case of sheep, among 214 ovaries CL was found in 78 ovaries with the remaining 136 ovaries having no CL.
3.1. Qualitative, Quantitative and Histological Parameters of Left and Right Ovaries
- The results of the different qualitative, quantitative and histological parameters of left and right ovaries in goat and sheep are summarized in Table 1. In this study, it was observed that all the qualitative and quantitative parameters along with histological measurements of different follicular aspects of left ovary were similar (P>0.05) to the right ovary in both goat and sheep (Table 1). The observation expressed that both left and right ovaries were equally active to normal physiological and/or ovarian activity. In accordance with the present study, Talukder et. al. [20] observed that there was no significant (P>0.05) difference in length, width and weight between the left and right ovaries of sheep. Islam et. al. [21] were worked on goat ovaries and expressed that the mean weight, length and width were found to be higher in right ovaries than those of left ovaries. They also expressed that the number of follicles, normal COCs and abnormal COCs were similar in both ovaries. They concluded that right ovaries were more active than left ones to show normal physiological and/or ovarian activity. But the results of some previous study, Singh et. al., [22] supports the present study that there was no significant difference (P>0.05) in the parameters of left and right ovaries of goat. There was no evidence of histological comparison in left and right ovaries of goat and sheep. Working with Iranian goat and sheep, Mohanunadpour [16] observed that there was no significant difference between the parameters of left and right ovaries in both species, which was similar with the results of the present study.
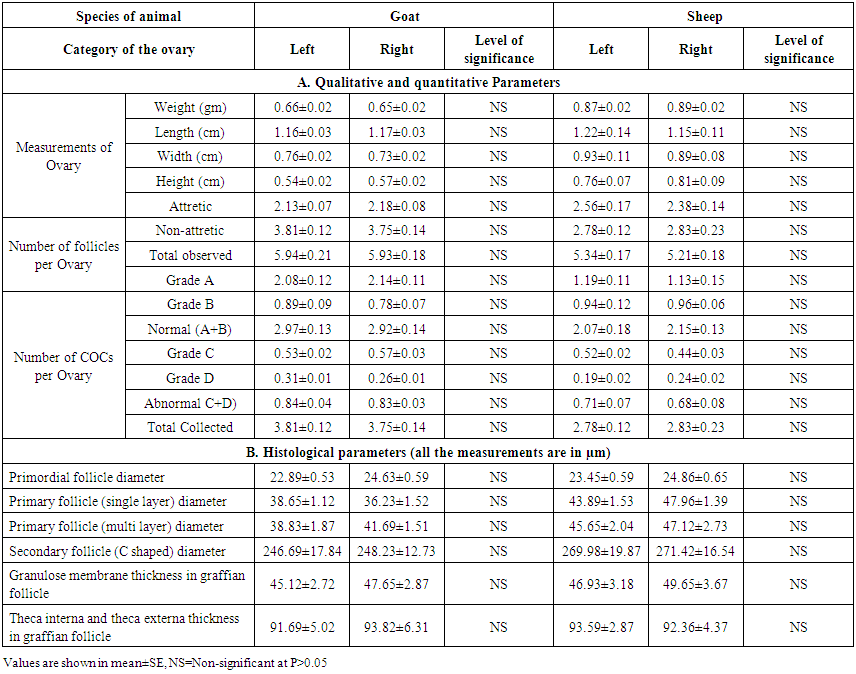 | Table 1. Qualitative, quantitative and histological parameters in right and left ovaries of goat and Sheep |
3.2. Qualitative, Quantitative and Histological Parameters of Ovaries with and without CL
- The comparative measurements of qualitative, quantitative and histological aspects of the ovaries with and without CL in goat and sheep are compiled in Table 2. It was observed that the mean weight, length, width and height of ovaries with CL in both goat and sheep were significantly higher (P<0.05) than those of ovaries without CL. As corpus luteum is an extra cellular material within the ovary which made the differences of its weight and dimensions found in this study. Islam et. al. [21] and Hoque et. al. [1] in goat and Talukder et. al. [20] in sheep were observed similar result that presence of CL increased the weight and dimensions of ovary. If we considered follicular health and number of follicle per ovary, it was observed that number of attretic follicle was significaly higher (P<0.05) in CL present ovary in both goat and sheep. Whereas, number of non-attretic follicle and total number of observed follicles were significantly lower (P<0.05) in CL present ovary than CL absent one irrespective of species (Table 2). In accordance with the present study, Jablonka-Shariff et. al. [23] expressed that as the hypertrophy of luteinized granulosa cells, hyperplasty of fibroblasts of the connective tissues and vascularity contribute to an increase in size of the CL, the increment of weight and dimensions along with follicular attretia of ovary was very usual. The present study also expressed that the number of normal (both grade A & B) and total COCs per ovary were significantly higher (P<0.05) in ovaries without CL type than CL present one in both goat and sheep. But the number of abnormal (both grade C and D) COCs per ovary was significantly higher (P<0.05) in present type than absent type of ovary (Table 2). The causes of higher number of normal and total follicles found in CL-absent group ovaries than those of CL group were understood well as it fits the endocrinological explanation. The result also similar with the observation of Islam et. al. [21], Hoque et. al. [1] as found in goat and Talukder et. al. [20] in sheep. By comparing the histological parameters between the CL present and absent type avary, it was observed that all the measurements of different follicles were similar (P>0.05) except the diameter of the Secondary follicle (C shaped) which was significantly higher (P>0.01) in ovaries without CL than with CL (Table 2). It is well established that all female mammals born with a large reservoir of follicles in ovary which rapidly decline as puberty approaches; but whether this early losses represent a mechanism of physiological wastage or not, is not definitely known. Follicle growth initiation is one of the most important and least understood aspects of ovarian biology and represents a major challenge for experimental study. Growth initiation of follicles has variously been attributed to i) hormonal triggers (gonadotropins) ii) stochastic processes (fluctuation in internal signal molecule) and iii) external inhibitory control from growing follicles [8]. The causes of similarities and differences among the follicular measurements is still unknown but it might be due to this hormonal activies of CL. The cause of lower yield of oocytes from the ovaries with CL may also be attributed to the fact that CL reduces the growth of follicles and increases the atresia of follicles [24]. In other way, due to the absence of CL, the negative effect of progesterone on anterior pituitary might not be functional in this ovary. So the higher number of COCs in this category other than CL functional group explains the role of hormonal balance on female folliculogenesis. Within the category, the higher number of normal COCs than that of abnormal COCs further supports the above statement. The findings of CL-absent group ovaries explain the role of progesterone on follicular degeneration and further strengthening the previous statement.
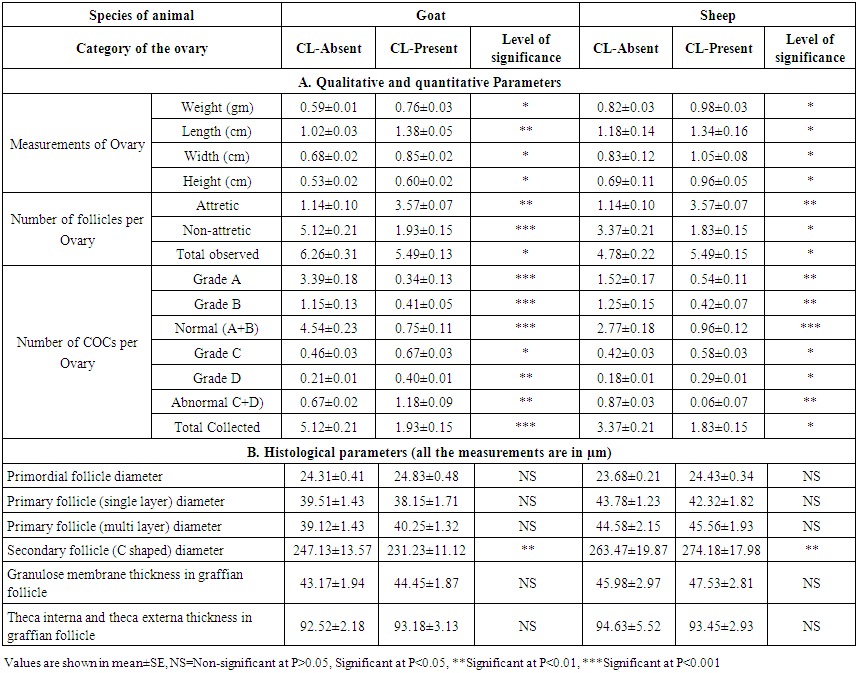 | Table 2. Qualitative and quantitative parameters in corpus luteum-present and -absent groups of ovaries of goat and sheep |
3.3. Interspecies Comparison of Qualitative, Quantitative and Histological Parameters of Goat and Sheep Ovaries
- Interspecies comparison of qualitative, quantitative and histological study between goat and sheep are summerized in table-3. It was observed that almost all of parameters in sheep were significant higher (p<0.05) than those of goat except number of abnormal COCs per ovary (Table 3). It was also observed that all the histological measurements of different follicles in sheep ovaries were higher than those of sheep but significantly higher (p<0.05) measurement was found in the diameter of primary follicle (multi layer), secondary follicle with C shaped antrum with C shaped antrum as well as the thickness of theca interna and theca externa in graffian follicles (Table 3). This is might be attributed to the interspecies variation in genetic, physiological and anatomical aspect of reproductive system between sheep and goat. Since the indigenous sheep of Bangladesh almost heavier in body conformation, accordingly the higher ovarian parameters in sheep might be attributed to this reason. In accordance with the present study, Mohanunadpour [16] reported that the differences of ovarian parameters between sheep and goat might be due to some qualitative and quantitative differences in circulating the hormones as well as the ovarian receptors for hormones of the two animals.
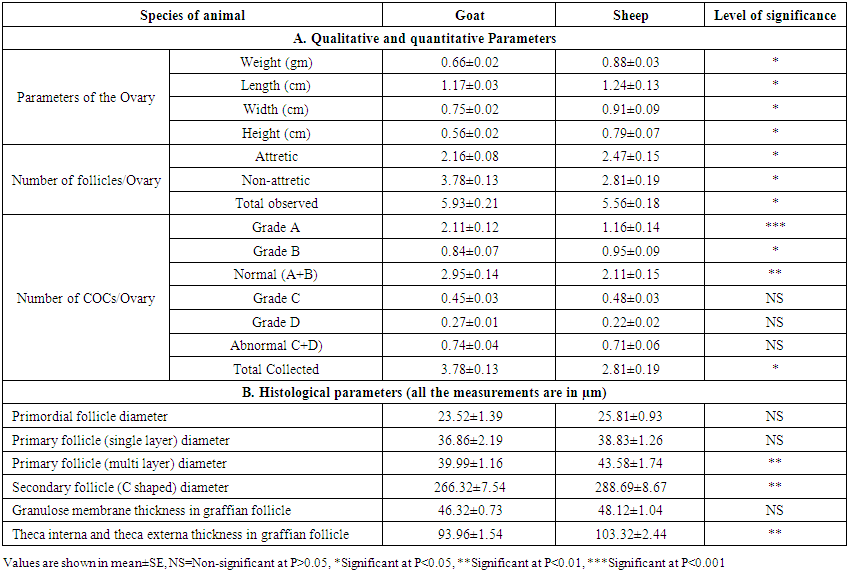 | Table 3. Comparison of qualitative and quantitative parameters between goat and sheep ovaries |
4. Conclusions
- After the above discussion, it can be concluded that ovaries with corpus luteum yielded significantly higher number of non-atretic follicles which resulted significantly higher number of normal and also total COCs used for IVM. The left and right ovaries are equally active for ovarian activity. The sheep ovaries were somewhat larger in size and possessed higher measurements in almost all aspect than those of goat ovaries. The findings of this study would be helpful for quality COCs collection for IVM and also in species documentation.
ACKNOWLEDGEMENTS
- We would like to acknowledge Research Management Committee (RMC), BSMRAU for providing the fund to conduct this piece of research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML