-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2016; 6(2): 45-48
doi:10.5923/j.als.20160602.03

A Study to Estimate Longevity of Thermostable Newcastle Disease Vaccine (strain I-2) in Village Chickens of Nepal
Shankar Nyaupane1, Bishwo B. Pokharel2, Madhav P. Acharya3
1Food and Agriculture Organization of the United Nations, Baitadi, Nepal
2University of Guelph, Guelph, ON, Canada
3Animal Health Research Division, Nepal Agriculture Research Council, Lalitpur, Nepal
Correspondence to: Shankar Nyaupane, Food and Agriculture Organization of the United Nations, Baitadi, Nepal.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

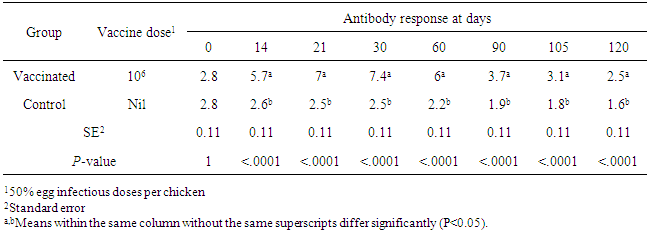
This study was conducted to estimate the longevity of thermostable Newcastle disease (ND) vaccine (strain I-2) in village chickens of Nepal. A total of 56 (27 day old) chicks were allocated randomly into 2 groups (treatment and control) with 28 birds in each group. On day 28, thermostable ND vaccine (strain I-2) was administered to the treatment group only. Blood samples were collected from experimental birds at 1 day prior to vaccination and 14, 21, 30, 60, 90, 105 and 120 days after vaccination. The serum obtained was titrated for NDV antibody using haemagglutination inhibition (HI) test. The log2 HI antibody titre level in vaccinated birds at 14, 21, 30, 60 and 90 days after vaccination were higher (P<0.05) compared to antibody titre level at 1 day before vaccination and control group. The log2 antibody titre at 14, 21, 30, 60 and 90 days after vaccination was higher than log2 HI titre level of ≥ 3 necessary to be protective against ND. There was no difference (P>0.05) in the antibody titre level in vaccinated birds at 1 day before vaccination and 105 days after vaccination suggesting that booster dose is required after 90 days of primary vaccination. Thus, thermostable ND vaccine (strain I-2) produced specific immunity against ND for at least 90 days after vaccination in village chickens of Nepal and may be considered suitable in Nepalese condition where cold chain maintenance is a huge challenge especially in rural area.
Keywords: Cold chain, Longevity, Newcastle Disease I-2 thermostable vaccine, Village chicken
Cite this paper: Shankar Nyaupane, Bishwo B. Pokharel, Madhav P. Acharya, A Study to Estimate Longevity of Thermostable Newcastle Disease Vaccine (strain I-2) in Village Chickens of Nepal, Advances in Life Sciences, Vol. 6 No. 2, 2016, pp. 45-48. doi: 10.5923/j.als.20160602.03.
Article Outline
1. Introduction
- With over seventy countries reporting Newcastle disease (ND) in domestic species [1] and many other countries with endemic Newcastle disease virus (NDV), ND has become a major killer and troubling viral disease in both commercial and backyard chicken. This disease is caused by an avian paramyxovirus serotype-1 which falls in the genus Avulavirus of Paramyxoviridae family [2]. In recent years, poultry farming has emerged as one of the major income source of Nepalese farmers contributing about 3.5% of the agricultural gross domestic product (GDP) of Nepal [3]. Rural poultry farming is the most widespread form of farming in Nepal with over 51% of households involving in this activity where they raise all species of poultry (especially local chickens) in the scavenging and backyard system [3]. Local chickens contribute about 16 percent of total eggs and 13.5 percent of chicken meat production in Nepal [4].Newcastle disease stands as a major problem towards the development of the poultry sector in Nepal as it is responsible for high economic losses due to high mortality, morbidity, stress, decreased egg production and hatchability [5]. Although humoral immunity from vaccination is critical in controlling ND [6], failure to maintain vaccine quality and sufficient cold chain has been a major problem in rural community of Nepal. Recent study about poultry industry of Nepal mentioned that vaccine failure is an important issue in poultry farming of Nepal suggesting rigorous research in thermostable vaccine of various diseases, including ND, Infectious Bursal Disease (IBD) and Avian Influenza (AI) [7]. Due to the lack of good transportation system in rural areas, it is not always feasible to construct a good cold chain to link vaccine producers to local chicken flocks. Also, it is difficult to institute good ND control system in a free-range poultry production system [8]. The thermostable ND vaccine was developed for vaccination of village chickens in developing countries where problem of cold chain maintenance is common [9, 10]. This vaccine has been widely used in African countries like Tanzania [11, 12], Uganda [8] and several other developing countries [13]. However, no study has been done yet to determine suitability of thermostable ND vaccine (NDV strain I-2) to local chickens of Nepal. Thus, the present study was conducted with the objective to estimate the longevity of thermostable ND vaccine (NDV strain I-2) in village chickens of Nepal.
2. Material and Methods
2.1. Experimental Design
- The study was conducted at the Animal Health Research Division (AHRD) of Nepal Agriculture Research Council (NARC), Lalitpur, Nepal for the duration of 5 months from 1st March to 5th August 2015. A total number of 65 one day old village chicks (local breed) were purchased and reared in experimental shed following the guidelines provided by NARC. All chickens were kept on deep litter and provided with ad libitum feed and water. On day 27, a total of 56 birds were randomly divided into two treatments including vaccinated (treatment) group and control group. On day 28, vaccinated groups received thermostable ND vaccine (I-2 strain) via intraocular route.
2.2. Vaccine and Vaccination
- On day 28, treatment group was vaccinated with thermostable ND vaccine (I-2 strain) via intraocular route at the dose rate of 0.1 ml/bird with each dose containing approximately 106 50% egg infectious dose (EID50) and control group was administered with phosphate buffer saline (PBS) via intraocular route at the dose rate of 0.1ml/bird. The vaccine was manufactured by the Central Biological Production Laboratory, Tripureshwor, and Kathmandu, Nepal. The vaccine was prepared from the master seed of the live lentogenic avirulent thermostable NDV (I-2 strain) imported from Department of Veterinary Pathology, University of Queensland, Australia. The vaccines were first diluted in 1 ml PBS and then added to 9 ml PBS such that 100µl makes 1 dose. The vaccines were stored according to the instructions provided by the manufacturer.
2.3. Serology
- Blood samples were collected from both treatment and control group 1 day prior to vaccination, 14 days, 21 days, 30 days, 60 days, 90 days, 105 days and 120 days after vaccination. A total of 3 ml of blood was collected from each experimental bird making a total of 448 blood samples. To obtain serum, blood collected in the sterile syringe was kept tilting at an angle of 30° to the horizontal surface for 24 hours. The serum collected were titrated for NDV antibody using the HI test [1].
2.4. Statistical Analysis
- The data obtained were log transformed to normalize the sample population for the validity of the test statistics applied. The log transformed data were then subjected to the GLIMMIX procedure of SAS (version 9.4, Inst. Inc. Cary, NC, USA) with individual bird considered as an experimental unit. The model countered for the fixed effect of vaccination and the antibody titre values were analysed as repeated measures with day as the repeated term. Linear and quadratic effects of day on the antibody titre level of vaccinated birds was determined using orthogonal contrasts. The inflection point for a quadratic response was determined by setting the first derivative to zero and solving for independent variable (x). The equation used was dy/dx = b1 + (2*b2x) where y is the response variable, x is the day, b1 is the linear component and b2 is the quadratic component of quadratic equation. Pairwise means comparison was done using a Tukey test. A value of P<0.05 was considered significant.
3. Results
- The relationships between vaccination of thermostable ND vaccine (I-2 strain) and antibody titre level of vaccinated birds at different days after vaccination are illustrated in Fig. 1. The antibody titre level increased quadratically in the vaccinated birds during day 14 and 30 after and then started to decrease during day 60 until day 120. At 120 days after vaccination, the log2 HI titre level dropped below 3. The inflection point was determined at day 49.8 where vaccinated birds had highest antibody titre level. The antibody titre level at 1 day prior to vaccination was found different (P<0.05) from antibody titre level at 14 days, 21 days, 30 days, 60 days, 90 days after vaccination but was not different (P>0.05) from 105 days and 120 days after vaccination.
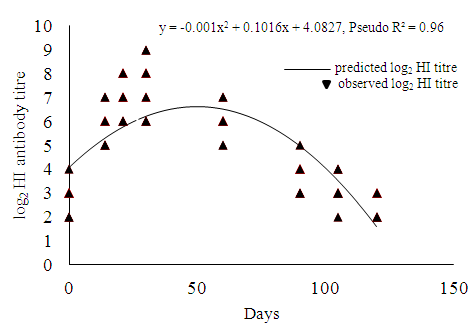 | Figure 1. Relationship between days after vaccination and log2 HI antibody titre in vaccinated birds |
|
4. Discussion
- The aim of this study was to investigate for how long thermostable ND vaccine (I-2 strain) can maintain adequate antibody titre in village chickens of Nepal. We found that vaccination of village chicken gave antibody of log2 HI titre of ≥3 from day 14 to day 105. It has been indicated that a high fraction of birds needs to have log2 HI titre of ≥3 to ensure no epidemic spread in the vaccinated population of birds [14]. Although vaccinated bird had antibody titre of >3 on day 105, it was not different from the antibody titre at 1 day prior to vaccination. This suggests that the booster dose of thermostable ND vaccine (I-2 strain) is required after 90 days of primary vaccination. This is in contrast with the study [9] which found that strain I-2 maintained adequate titres for 9 months in a small flock of chickens kept under village conditions. The antibody titre level of control birds in this study was found to be 2.28 ± 0.096 which is higher than the previous study done in commercial and village chickens of Tanzania [11]. In the same study, higher antibody levels in vaccinated birds at 14th (8.2), 60th (5.1) and 90th (4.2) days after vaccination. This is in agreement with our study. Our study suggested sufficient protection level against NDV until 90 days of primary vaccination. This is very important for rural poultry farmers of Nepal with minimal transportation facilities as they can hardly provide good refrigeration during transport. This finding helps rural farmers to plan their vaccination schedule. High cost of commercial heat-resistant ND vaccine and availability in 1000-dose ampules only is hindering the use of other commercial vaccines available in Nepal. This study opens the door of opportunity and is helpful in addressing the vaccines issues in Nepal which are specifically related to cold chain maintenance.As ND is a constant threat to village chickens of Nepal, the need of thermostable vaccine is even more important. This vaccine has higher shelf-life in ambient room temperature and can be produced in the local veterinary laboratories with the requirement of use within two weeks of production [11, 13]. Furthermore, this vaccine is suitable to be administered with food [15]. Wide applicability and easy adaptability of thermostable ND vaccine has proved its essence among rural poultry farmers of Nepal.
5. Conclusions
- In conclusion, thermostable ND vaccine (strain I-2) produced specific immunity for at least 90 days of primary vaccination suggesting the need of the booster dose after 90 days of primary vaccination. Thermostable ND vaccine can be considered suitable for application in village chickens of Nepal reared under free-range system. A more detailed field study involving both commercial and village chickens of Nepal is recommended in the future.
ACKNOWLEDGEMENTS
- This study was financially supported by the Institute of Agriculture and Animal Science, Tribhuvan University (TU), Agriculture and food security project (AFSP) of the Nepal Government, Food and Agriculture Organization of the United Nation (FAO). The authors thank all the people who assisted during the experimental process.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML