-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(4): 85-89
doi:10.5923/j.als.20150504.02

Cysteic Acid Formation Behaviors in Bleached Hair of Southeast Asian Characterized by Infrared Spectroscopy
Kosuke Watanabe , Keiko Nagami , Kazuyuki Suzuta , Takaaki Maeda , Len Ito
Central Research Institute, Milbon Co. Ltd., Osaka, Japan
Correspondence to: Kosuke Watanabe , Central Research Institute, Milbon Co. Ltd., Osaka, Japan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bleaching is one of hair-decoloring techniques widely used in the beauty treatment. Since hydrogen peroxide is used in this process, disulfide bonds in proteins, which represent a major class of hair components, are oxidatively cleaved and cysteine residues are converted to cysteic acid residues. Increased cysteic acid is considered to be a major cause of hair damages, and local changes in mechanical properties through cysteic acid formation have been presumed to affect the hair texture in an undesirable manner. In this study, we characterized the cysteic acid formation in Southeast Asian hair multidirectionally by means of three infrared spectroscopy-based techniques (ATR, KBr tablet, and microscopic). The result obtained by the ATR method showed that the amount of cysteic acid increase in a stepwise manner with respect to bleaching time. Since the measurement region of the ATR method is the cuticle layer on the hair surface, the KBr tablet method was used to analyze the cysteic acid formation behaviors in the cortex, an inner layer of the hair shaft. The result showed a stepwise increase depending on bleaching time, similarly to the result from the ATR analysis. Further, the result from microscopic analysis using the large synchrotron radiation facility SPring-8 demonstrated that the amount of cysteic acid formed by bleaching was constant across the cortex layer. Detailed analysis of the data obtained with these three methods suggested that the diffusion rate-limiting kinetics of cysteic acid formation in the cortex layer was due to slow diffusion of hydrogen peroxide from cell membrane complexes into cortex cells.
Keywords: IR, Hair, Cysteic acid, Synchrotron radiation, Southeast Asian
Cite this paper: Kosuke Watanabe , Keiko Nagami , Kazuyuki Suzuta , Takaaki Maeda , Len Ito , Cysteic Acid Formation Behaviors in Bleached Hair of Southeast Asian Characterized by Infrared Spectroscopy, Advances in Life Sciences, Vol. 5 No. 4, 2015, pp. 85-89. doi: 10.5923/j.als.20150504.02.
Article Outline
1. Introduction
- Hair coloring is an important technique in the cosmetic industry as a method widely used mainly by beauty salons to create various hair styles. In case of typical hair coloring technique, color of hair can be changed by simultaneously causing one reaction of oxidative decolorizing for melanin pigments and the other to develop a color by an oxidative polymerization of colorless aromatic amine compound called oxidative dye [1]. The former decoloring reaction is called as “Bleaching.” These reactions are enhanced by oxidation agents such as alkaline hydrogen peroxide. Hair consists of two major morphological tissues, i.e. cuticle and cortex. Cuticle is composed of overlapped plate-like cuticle cells at the outermost layer of hair, whereas cortex is composed of aggregate of spindle-shaped cortical cells. These cells are bonded with each other via cell membrane complex (CMC). Cortical cell is comprised of intermediate filament (IF) and globular matrix protein (KAP). It is well-known that bleaching treatment based on hydrogen peroxide for hair fiber with such complicated hierarchical structure may cause oxidative decolorization of melanin pigment and excessively oxydized cysteine as a prime constituent amino acid of the hair keratin at the same time resulting in creation of byproduct of cysteic acid [2-4]. It has been reported that cleavage of the disulfide (SS) bonds accompanied by the cysteic acid generation within the hair keratin may cause elution of protein [5]. Mechanical property deterioration by bleaching treatment has been also frequently reported as a physical change of fiber [6, 7]. As such mechanical deterioration is interpreted as caused by oxidative cleavage of SS bonds coinciding with cysteic acid generation, increase of cysteic acid content is considered to be the main cause of hair damage and therefore, amount of cysteic acid generated is suggested as a barometer of degree of hair damage [2, 8]. Edman and Marti described the change in the 20 % index of hair fibers as a function of treatment time in 6 % hydrogen peroxide at 32°C using a 25: 1 solution-to-hair ratio at pH 9.5 [6]. Relationship between their data and the square root of treatment time provided a straight line [9], suggesting that the oxidation of the disulfide bond in hair by alkaline hydrogen peroxide is a diffusion-controlled reaction. However, little is known about the diffusion mechanism of hydrogen peroxide and the generation behavior of cysteic acid inside the hair fiber for supporting the diffusion-controlled behavior. IR spectroscopy has been a mainstream up to now for evaluation of cysteic acid due to its relative easiness for the measurement [10-12]. IR microspectrometry is used for a cross-sectional slice of the hair fiber prepared by microtome aiming at evaluation of cysteic acid inside the hair [13]. In recent years, local distribution of lipid and cysteic acid has been studied based on IR microscopy using synchrotron radiation source with high luminance and excellent directive property [14, 15]. In the present study, we have attempted to evaluate the local distribution of cysteic acid generated in hairs for which bleaching treatment was performed for various periods of time using IR microscopic method with the synchrotron radiation source, and to understand the cysteic acid generation behavior caused by bleaching treatment through the comparison with the results from Attenuated Total Reflection (ATR) and KBr tablet methods.
2. Experiment
2.1. Specimens and Reagents
2.1.1. Purification of Hair
- Bundles of hairs with a length of about 25 cm and a weight of 1 g each were prepared from chemically unmodified hairs with length of about 30 cm collected from a female Southeast Asian. The hairs were purified by washing with distilled water and air-drying after immersion in 5 % laureth-9 solution containing 20 mM of EDTA at a room temperature for 1 hour.
2.1.2. Reagents
- As hydrogen peroxide, commercially available 35 wt % solution was used. Commercially available 25 wt % aqueous ammonia was also used. A reagent for IR absorption measurement was adopted for potassium bromide used for KBr tablet method.
2.2. Bleaching Treatment
- Bleaching treatment was performed by soaking the bundles of hairs in 3 wt % hydrogen peroxide solution adjusted at pH 10.4 by aqueous ammonia by a bath ratio of 1: 100 at a room temperature for 15, 30, 60, 120, 240, and 360 minutes. Finally, the bundles of hairs were sufficiently rinsed with purified water and air-dried.
2.3. IR Spectral Measurement
2.3.1. ATR Method
- In ATR method, measurement was performed using Nexus 670 model of Thermo Nicolet under conditions of 128 times of scanning and resolution of 4 cm-1. Incidence angle of 45° was adopted for measurement using a Ge prism with high refractive index. ATR collection was performed for resulting spectrums by assuming the refractive index of cuticle to be 1.55 [16].
2.3.2. KBr Tablet Method
- KBr tablet measurement was performed according to usual method. Specimens for infrared absorption spectrometry were prepared by mixing 0.5 mg of hair fibers finely cut by scissors with 50 mg of dried powder potassium bromide in an agate mortar, pulverizing promptly not to absorb moisture and forming in a tablet forming machine (MHP-1 of Shimadzu Co.). Nexus 670 model of Thermo Nicolet was also used for infrared absorption spectrometry similarly to the ATR method. Measurement was performed under conditions of 128 times of scanning and resolution of 4 cm-1.
2.3.3. IR Microscopic Method
- For experiments based on microscopic IR method, we used an infrared spectrometer (Vertex 70 model of Bruker) combined with infrared microscope (Hyperion 2000 of Bruker) installed at a beamline BL43IR of large-scaled synchrotron radiation facility SPring-8 (Super Photon ring-8GeV) located in Japan. Hair cross-sectional slices with a thickness of 10 μm were prepared with the hair fibers frozen in distilled water by using cryo-microtome. Transmission measurement was performed for slices put on a barium fluoride plate by setting them on a mapping stage of infrared microscope. Based on an aperture size of 5 μm × 5 μm, measurement was performed for random sites within the hair cross-sections under conditions of 128 times of scanning and resolution of 4 cm-1.
3. Result and Discussion
3.1. Infrared Absorption Spectrum of Hair
- Based on measurements of infrared absorption spectrum for untreated and bleached hairs by ATR, KBr tablet, and IR microscopic methods, typical spectrums normalized by absorption at 1650 cm-1 corresponding to amide I are shown in Fig. 1. A lot of absorption peaks observed within 800 to 4000 cm-1 wavenumber range are attributed according to chemical structure of hair keratin [17, 18]. The characteristic absorption peaks at 1175 cm-1 and 1040 cm-1 for the bleached hair are derived from sulfonate group of cysteic acid generated by oxidation of cysteine residue as S=O antisymmetric stretching vibration and S=O symmetric stretching vibration [19], respectively.
3.2. Amount of Cysteic Acid Generated by Bleaching Treatment in Cuticle and Cortex
- In general, amount of cysteic acid within keratin fiber is estimated semiquantitatively based on absorption peak intensity at 1040 cm-1 [13, 14]. Cysteic acid generation behavior was analyzed using an absorption peak at 1040 cm-1 measured by ATR and KBr tablet methods. Drawing a linear baseline from 1030 cm-1 to 1060 cm-1 for the spectrum normalized by amide I at 1650 cm-1, amount of cysteic acid was estimated as a peak height from baseline at 1040 cm-1, I1040. This baseline corresponds to an absorption spectrum of hair fiber without any cysteic acid. Relationship between I1040 and time of bleaching is shown in Fig. 2. In both ATR and KBr tablet methods, such tendency was clearly observed that amount of cysteic acid increases as the bleaching time increases. As the penetration depth of infrared light by ATR method used in the study was around 1 μm and the measuring position is considered to be in the cuticle, it can be considered that the amount of cysteic acid within cuticles increases with the lapse of bleaching time. In contrast to ATR method which reflects chemical structure of cuticle, KBr tablet method as the transmission measurement for pulverized hairs may be approximately regarded to reflect chemical structure of cortex which makes up most of hair. The result of the amount of cysteic acid observed in KBr tablet method means increase in the amount of cysteic acid in cortical cells with increasing time of bleaching. In consideration of a slight content of cysteic acid for untreated hair, the value of I1040 for untreated hair may not become 0 by both ATR and KBr tablet methods. This value increased with the bleaching time and the value of hair with 360 minutes of treatment increased up to about 2.5 times of that of untreated hair.
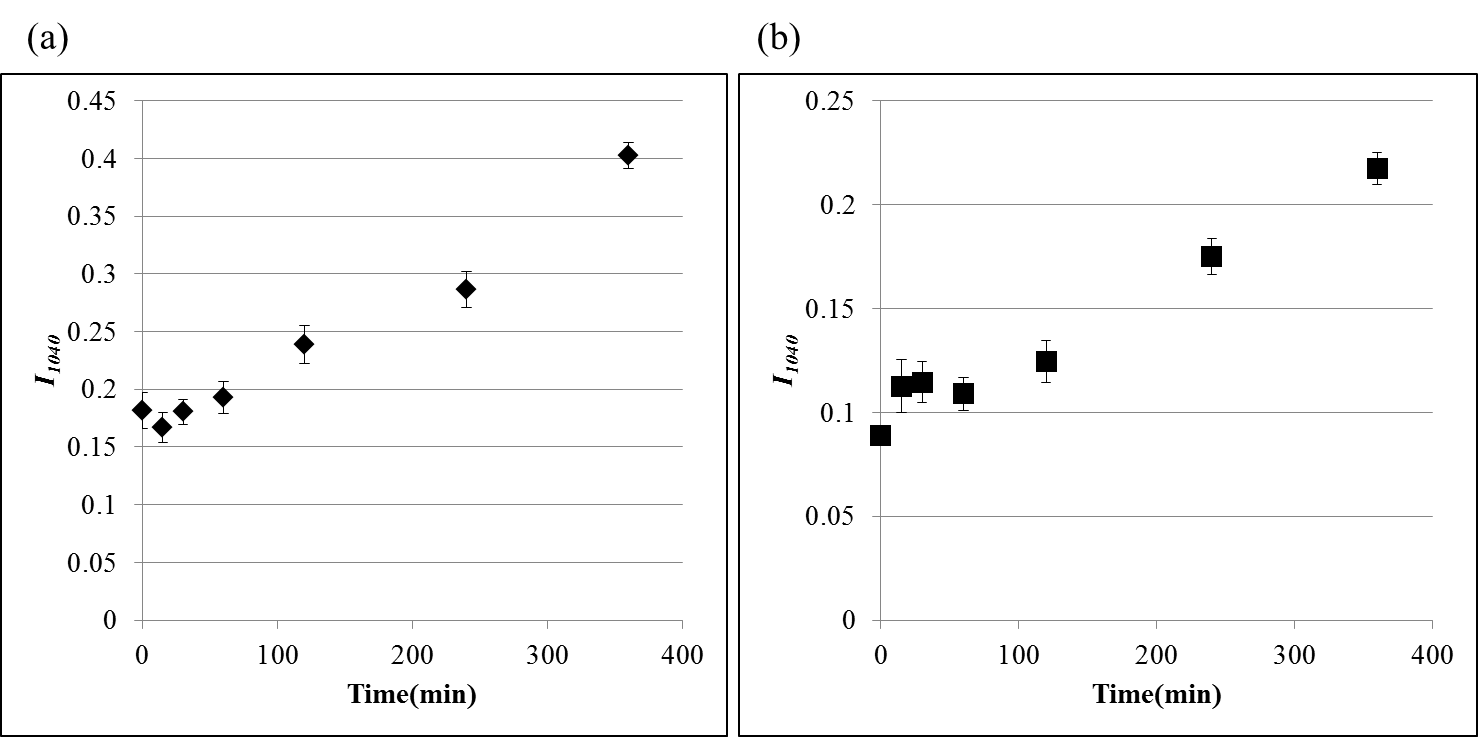 | Figure 2. Relationship between bleaching time and peak height at 1040 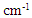 , I1040. (a) ATR method, (b) KBr tablet method (N = 10 in both methods) , I1040. (a) ATR method, (b) KBr tablet method (N = 10 in both methods) |
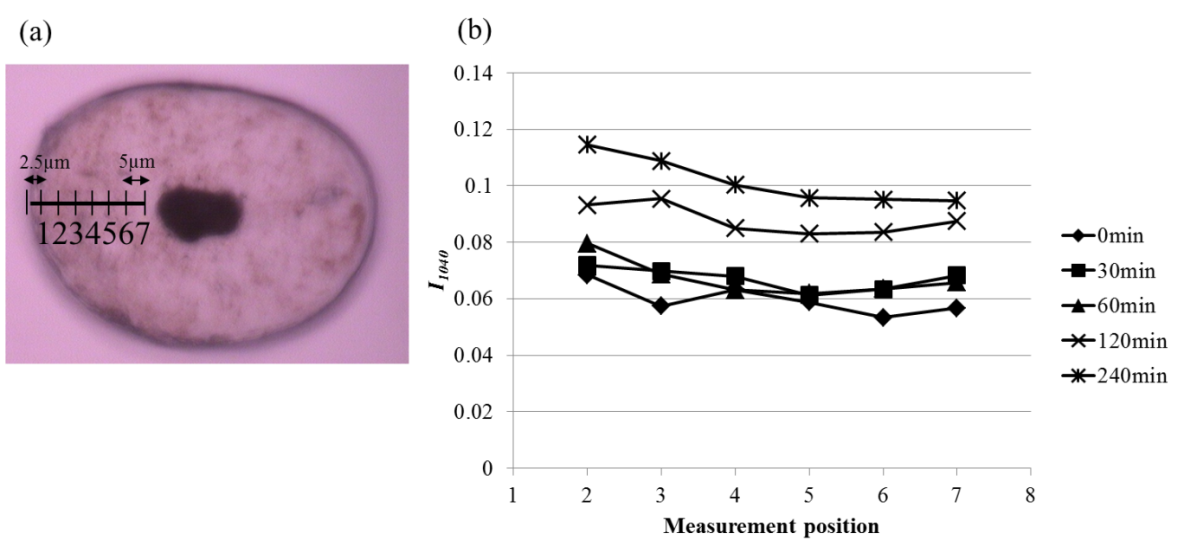
3.3. Local Analysis of Cysteic Acid using IR Microscopic Method
- We have analyzed cysteic acid generation behavior using ATR and KBr tablet methods. However, localization of cysteic acid generation inside hair has hardly been clarified.Therefore, IR microscopic measurement was performed at a large-scaled synchrotron radiation facility, SPring-8, in order to obtain information about local generation behavior of cysteic acid inside the hairs. As shown in Fig. 3(a), seven measurement points were set with an interval of 5 μm from any point of about 2.5μm depth by hair surface toward central point for hair cross-section prepared by a microtome. In analyzing spectrums obtained by measurement, baseline of cuticle site, measurement point of one, was bending with large inclination of the spectrum as a whole (data not shown). As it was considered to have been possibly affected by increased scattering and interference of infrared light due to multilayered structure of cuticles, measurement was performed within the cortex area except cuticle site of measurement point of one in the study to determine amount of cysteic acid generation. Based on measurement of infrared absorption spectrums at each measuring point, peak height at I1040 was obtained by normalization using amide I at 1650 cm-1 upon setting the baseline as mentioned above. The values of I1040 at each measuring point are shown in Fig. 3(b). The values of I1040 at each measuring point are lowest for untreat hair and increase with the lapse of bleaching time. It indicates, as shown also by ATR and KBr methods, that the amount of cysteic acid in hair tends to increase with the increase of bleaching time. In addition, it has been found out that the values of I1040 at all measuring points located within the cortex area were almost constant for each of hair samples. In general, hydrogen peroxide is diffused over the whole hair through cell membrane complex which is spread out over the whole cuticles and cortex. Further, it is considered that cysteic acid is generated as a result of oxidation of SS bonds in IF and KAP protein caused by hydrogen peroxide diffusion from cell membrane complex to cortical cells within the cortex area. The uniform oxidation behavior shown in Fig. 3(b) may be considered to indicate that the rate of cysteic acid generation by SS bond oxidation is mainly controlled by diffusion of hydrogen peroxide from cell membrane complex into cortical cells because diffusion rate of hydrogen peroxide into cortex area through cell membrane complex is much higher.
4. Conclusions
- In the present study, we have examined cysteic acid generation behavior for bleached hairs using infrared spectroscopy. From results of ATR and KBr tablet methods, it has been proved that amount of cysteic acid increases gradually in cuticle and cortex with increasing bleaching time. In addition, it has also been revealed that amount of cysteic acid generation is uniform within the cortex area by IR microscopic measurement using synchrotron radiation source. The uniform oxidation behavior may be considered to indicate that the rate of cysteic acid generation by SS bond oxidation is mainly controlled by diffusion of hydrogen peroxide from cell membrane complex into cortical cells because diffusion rate of hydrogen peroxide into cortex area through cell membrane complex is much higher.
ACKNOWLEDGMENTS
- The synchrotron radiation experiments of the present study, as research proposals of 2012B1385, 2014A1563, 2014B1591 and 2015A1654 that has been adopted by the Japan Synchrotron Radiation Research Institute, were performed at the BL43IR beam line of SPring-8. We deeply thank Dr. Taro Moriwaki and Dr. Yuka Ikemoto, the Japan Synchrotron Radiation Research Institute, for their support of our experiment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
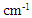 . The arrowed line and arrowhead indicates 1178
. The arrowed line and arrowhead indicates 1178 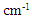 and 1040
and 1040 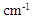 , respectively
, respectively