-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(4): 73-84
doi:10.5923/j.als.20150504.01

Dysfunction of nM Ouabain-Induced Activation of the Signaling System Responsible for Age-Related Heart Muscle Failure
Lilia Narinyan, Sinerik Ayrapetyan
Life Sciences International Postgraduate Educational Center, UNESCO Chair, Yerevan, Armenia
Correspondence to: Sinerik Ayrapetyan, Life Sciences International Postgraduate Educational Center, UNESCO Chair, Yerevan, Armenia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cell dehydration is one of the essential hallmarks for aging. Although Na+/K+-ATPase has a crucial role in metabolic regulation of cell dehydration, the individual role of three catalytic isoforms of Na+/K+-ATPase (α1, α2 and α3) in generation of age-induced cardiac muscle dehydration is not elucidated yet. It is known that these isoforms have different functions and affinities to ouabain (specific inhibitor for Na+/K+-ATPase). In our previous study we have shown that the activation of α3 receptors by ≤10-9 M ouabain brings to activation of cAMP-dependent Na+/Ca2+ exchange in reverse mode (R Na+/Ca2+ exchange), which has age-dependent depression character. The aim of the present work was to study the role of nM ouabain–activated Na+/Ca2+ exchange in age-dependent cardiac muscle dehydration. For this purpose the age-dependency of [3H]-ouabain binding with cell membrane and the effect of the latter on muscle hydration, 45Ca2+ uptake and 45Ca2+ efflux in heart muscle of young and old ratsinvivo and invitro conditions were studied. Age-dependent decrease of cardiac muscle hydration was accompanied by the decrease of dose-dependent [3H]-ouabain binding with α3 receptor. The activation of the latter by ≤10-9 M ouabain had stimulation effects on 45Ca2+ uptake, which was accompanied by muscle hydration. This stimulation had age-dependent weakening character and was depressed invitro conditions at 7oC. The rate of 45Ca2+ efflux from heart muscle had strong age-dependent depressing character. 10-9 M ouabain stimulated the rate of 45Ca2+ efflux from heart muscles of both ages of rats and also had pronounced age-dependent weakening character. The fact that ≤10-9M ouabain-induced activation of R Na+/Ca2 exchange, functioning in stoichiometry of 1Ca:3Na was not accompanied by muscle dehydration speaks about the crucial role of intracellular signaling system controlling both fibrillar Ca2+-dependent contractility and the rate of endogenous H2O release as a result of oxidation processes in regulation of myocyte hydration. Therefore, it is suggested that age-induced dysfunction of Na+/K+-ATPase α3 isoform-dependent signaling function is a primary mechanism for muscle dehydration and increase of [Ca2+]i which leads to heart muscle failure.
Keywords: Water content, α isoforms, Signaling function, Muscle contractility
Cite this paper: Lilia Narinyan, Sinerik Ayrapetyan, Dysfunction of nM Ouabain-Induced Activation of the Signaling System Responsible for Age-Related Heart Muscle Failure, Advances in Life Sciences, Vol. 5 No. 4, 2015, pp. 73-84. doi: 10.5923/j.als.20150504.01.
Article Outline
1. Introduction
- Cell hydration (water content) is a fundamental parameter determining functional activity of cell. It is realized by changing the activity of intracellular macromolecules via “folding-unfolding” mechanism [1] as well as by cell surface-dependent changes of the number of functionally active protein molecules in plasma membrane which has enzymes, receptors and ion channels forming properties [2-4]. Cell dehydration is one of the essential hallmarks for aging [5-10]. Therefore, the dysfunction of metabolic regulation of cell hydration could serve as one of the primary mechanisms for generation of cell pathology, including aging.Although the important physiological role of water in cell functional activity is widely recognized, its messenger role in generation of various diseases [11-14] has not been clarified yet. Therefore, the elucidation of the detailed mechanism responsible for regulation of cell hydration could help understand the role of cell hydration in generation of age-related medical disorders, including heart muscle failure.As cell membrane is highly permeable for water, cell hydration is controlled by metabolic activity of cell. The latter is realized by the following two pathways: a) transporting mechanism of membrane controlling the number of osmotically active particles in cytoplasm, b) intracellular signaling system controlling absorption properties of cytoplasm and generation of endogenous water molecules in cytoplasm during oxidative phosphorylation. However, the dysfunction of which aforementioned pathways is the primary mechanism for generation of age-dependent heart muscle dehydration is not clear yet.As heart muscle has pacemaker contractility, it has high rate of adenosine triphosphate (ATP) utilization leading to stimulation of ATP synthesis, which is accompanied by the release of endogenous H2O in cytoplasm. It is known that 42 water molecules are released in cytoplasm as a result of one molecule of glucose oxidation [15]. Therefore, the release of endogenous H2O could have an essential role in metabolic control of cell hydration and its dysfunction could be one of the mechanisms responsible for heart muscle failure.The next fundamental parameter of cell, which is determinant in heart muscle contractility is intracellular calcium ions ([Ca2+]i). It is well established that aging leads to the increase of [Ca2+]i [16-20]. However, the detailed mechanism of close-talking interaction between cell dehydration and the increase of [Ca2+]i in aging has not been elucidated yet.The dysfunction of Na+/K+-pump, which is a common consequence of aging, has a key role in metabolic regulation of both cardiomyocyte hydration and [Ca2+]i homeostasis. Na+/K+-pump, being a high metabolic energy (ATP) utilizing mechanism and working with high intensity in cardiomyocytes, has a great intracellular signaling role in controlling Ca2+ sorption properties of intracellular structure as well as in generation of endogenous H2O in cytoplasm. Therefore, Na+/K+-pump could be considered not only as an ion transporting mechanism but also as a powerful intracellular signaling system controlling cell hydration and [Ca2+]i in myocytes. At present, it is well established that Na+/K+-ATPase (working molecules of Na+/K+-pump) in myocyte membrane has three catalytic isoforms (α1, α2, α3) with different affinities to ouabain (specific inhibitor for Na+/K+-ATPase) and with different functional activities [21-25]. However, the individual roles of these isoforms in regulation of myocyte hydration and [Ca2+]i require further elucidation.It is established that α1 (low affinity) and α2 (middle affinity) isoforms are involved in ion transporting processes, while α3 (high affinity) has only intracellular signaling function [22, 25-28]. Although α3 isoform is not involved in the function of transporting Na+ and K+, it has a crucial role in regulation of Na+/Ca2+ exchange [21, 25, 29-33]. By our previous study it has been shown that α3 isoform-dependent myocyte hydration and its affinity to ouabain have more pronounced age-dependent dysfunctional character than those in case of α1 and α2 [34]. However, the role of α3 isoform in determination of age-dependent myocyte dehydration and increase of [Ca2+]i is not clear yet. It is suggested that the elucidation of the mechanism(s) through which α3 isoform regulates myocyte dehydration and increase of [Ca2+]i will allow us to understand the role of age-dependent dysfunction of α3 isoform in heart muscle failure. For this purpose, in present work the following age-dependent studies have been performed both in vivo and in vitro experiments: determination of dose-dependent [3H]-ouabain binding with cell membrane and muscle hydration, measurement of 45Ca2+ uptake and efflux.
2. Materials and Methods
2.1. Animals
- Studies were carried out on young (6 weeks) and old (12 months) male Wistar albino rats of mass 50-60 g and 215-230 g, respectively. Animals were kept in a specific pathogen-free animal room. In present experiments the rats (N=470) were housed under optimum conditions with 12 h light/dark cycle at 22 ± 2°C and were given a sterilized diet and water ad libitum. All procedures performed on animals were carried out following the protocols approved by the Animal Care and Use Committee of Life Sciences International Postgraduate Educational Center (Yerevan, Armenia).
2.2. Chemicals
- Tyrode’s physiological solution (PS) with the following composition was used (in mM): 137 NaCl, 5.4 KCl, 1.8 CaCl2, 1.05 MgCl2, 5 C6H12O6, 11.9 NaHCO3, 0.42 NaH2PO4 and adjusted to pH=7.4. A radiometer PHM-22r (Radiometer, Copenhagen, Denmark) was used for pH measurements.In order to receive PS with 50% [Na+], 68.5mM of NaCl was replaced with 2M of non-metabolizing substance mannitol dissolved in Tyrode’s PS for maintaining the osmolarity of solution. All chemicals were obtained from “Medisar” Industrial Chemical Importation Company (Yerevan, Armenia).[3H]-ouabain (specific activity 25.34 Ci/mM) and cold (non radioactive) ouabain were obtained from Perkin Elmer (Waltham, MA, USA). All doses of ouabain were prepared on the basis of the physiological solution and used for intraperitoneal injection (in vivo) or heart muscle tissue incubation (in vitro).45Ca2+ (with specific activity of 40 mCi/ml) was obtained from Perkin Elmer (Waltham, MA, USA) and was used for in vivo and in vitro experiments. The study of 45Ca2+ uptake and efflux in heart muscle tissue was performed on young and old animals. For this purpose in Tyrode’s physiological solution (PS) containing 1.8 mM CaCl2, 0.0115 mM was substituted by labeled 45CaCl2. In the experiments that were aimed at studying 45Ca2+ uptake and efflux, all concentrations of ouabain were prepared with this radioactive PS.
2.3. Tissue Preparation
- To avoid an anesthetic effect on initial functional state [35-36] in present experiments we preferred to use sharp freezing method [37]. Animals were immobilized by dipping their heads into liquid nitrogen (for 3-4 s) and then they were decapitated. After decapitation of animals, their hearts were immediately placed in the tube with PS, and then six pieces were taken from each tested heart muscle with 50-60 mg wet mass (w. m.) per piece (time interval between these two procedures was not more than 30 sec).In vivo experiments, 30 min before decapitation animals were intraperitoneally injected by investigated solutions. After this they were immobilized and decapitated. In vitro experiments animals were firstly immobilized and decapitated. Their heart muscle tissues were dissected in the same manner and then incubated in investigated solutions.For removing surface-adherent and extra-cellular tracers (3[H]-ouabain and 45Ca2+), the samples were washed three times with PS in all experiments. After that the wet mass (w.m.) of samples was determined (time duration for these processes was less than 1 min for 30 samples). Similar procedures were performed with the samples of control and experimental groups.
2.4. Definition of Heart Muscle’s Water Content
- Determination of the water content (hydration) of heart muscle tissues was performed by traditional “tissue drying” method [38]. After determination of wet mass, the samples were dried in thermostat (Factory of Medical Equipment, Odessa, Ukraine) during 24 h at 105°C in order to estimate water content in muscle samples. The quantity of water in 1 g of dry mass (d. m.) of tissue was derived by the following equation: (w .m. – d. m.) / d. m.
2.5. Counting of [3H]-ouabain Receptors in Membrane
- Radioactive [3H]-ouabain is usually used to estimate the number of Na+/K+-pump units in membrane. It is assumed that each binding site in the membrane binds one molecule of ouabain [39]. After 30 min of [3H]-ouabain injection (in vivo experiments) the rats were decapitated and heart muscle samples were washed three times (10min-5min-5min) with normal Ringer's solution to remove surface-adherent and extra-cellular tracer. In vitro experiments after decapitation of rats the heart muscle samples were incubated in [3H]-ouabain for 30min and then washed three times (10min-5min-5min) with normal Ringer's solution. After determination of water content by the method described previously, dried tissue samples were replaced into special tubs and homogenized in 50 µl 68% HNO3 solution. Finally, 2 ml of of Bray’s scintillation fluid was added and the radioactivity of samples was calculated as counted per minute (CPM)/mg by Wallac 1450 liquid scintillation and luminescence counter (WallacOy, Turku, Finland).
2.6. Definition of 45Ca2+uptake and Efflux in Heart Muscle Tissues
- 45Ca2+ uptake was measured in vivo as well as in vitro conditions. In vivo experiments in Tyrode’s physiological solution 0.0115 mM CaCl2 from 1.8 mM was substituted by 45Ca2+. Young and old animal groups were intraperitoneally injected with 45Ca2+ (with 0.187 mCi/g radioactivity of body weight). After 30 min animals were decapitated and heart muscle samples were incubated for 30 min in PS (as a control) and PS containing different doses of ouabain. Then all samples were dried in thermostat during 24 h at 105°C.In vitro experiments heart muscle tissue samples were incubated in 20 ml cold (7°C) K+-free PS containing 1.8μl 45Ca2+ (as a control) or in PS containing different doses of ouabain for 30 min. Then they were dried in thermostat for 24 h at 105°C.The study of 45Ca2+efflux from preliminarily 45Ca2+-enriched heart muscle tissue was performed on young and old rats. For enriching heart muscle tissues by 45Ca2+ they were incubated for 1 h in 16.25 ml K+-free (containing 50% NaCl) physiological solution. In K+-free solution containing 1.8 mM CaCl2, 0.00448 mM was replaced by labeled 45CaCl2. Then enriched samples were washed three times in K+ free solution (containing 100% NaCl and cold CaCl2) for 10 min, 5 min and 5 min, respectively, to remove 45Ca2+ from extra-cellular spaces. The samples were divided into two parts. The first 30 samples (control) were dried in thermostat for 24 h at 105°C after determination of wet mass. The second set of 30 samples was incubated for 30 min in 20 ml of PS or in solutions containing different doses of ouabain and after determination of wet mass all samples were dried in thermostat for 24 h at 105°C. After determination of dry mass, all samples homogenized in 50 µl of 68% HNO3 solution, and the radioactivity of the samples was measured as cpm/mg dry weight. The rate of 45Ca2+ efflux was calculated as the residual part of absorbed 45Ca2+ for control and the experimental data by the following equation:[control – exp.]/control.
2.7. Statistical Analysis
- The mean and standard error of the heart muscle hydration index, [3H]-ouabain binding and 45Ca2+ changes in different samples were calculated and the statistical probability was determined by Student's paired t-test by means of computer program Sigma Plot (Version 8.02A, San Jose, CA,USA).The statistical probability was reflected in figures by asterisks (*). For all statistical tests P value was taken as *P < 0.05; **P < 0.01; ***P < 0.001.
3. Results
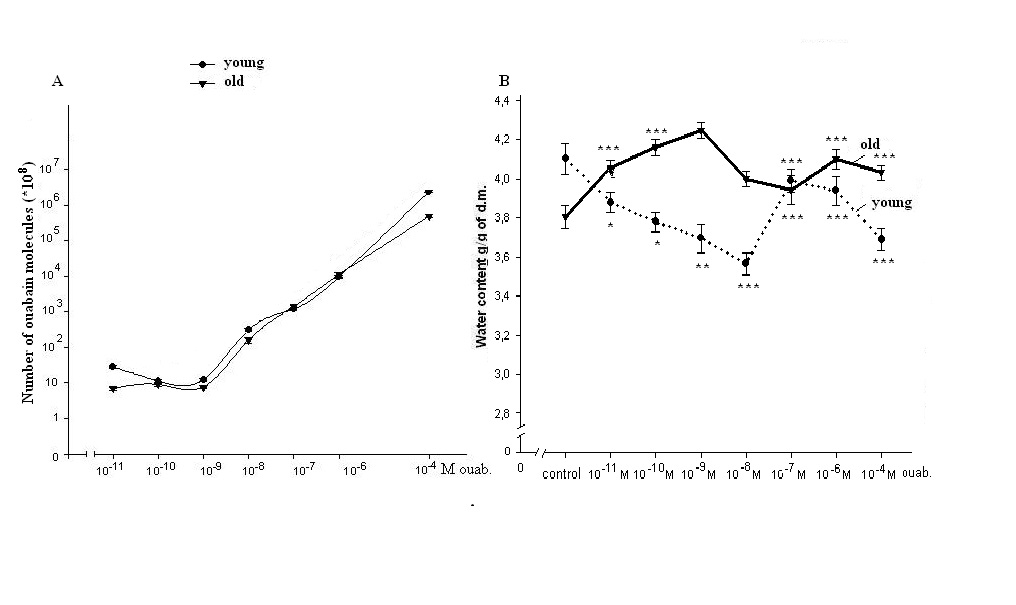
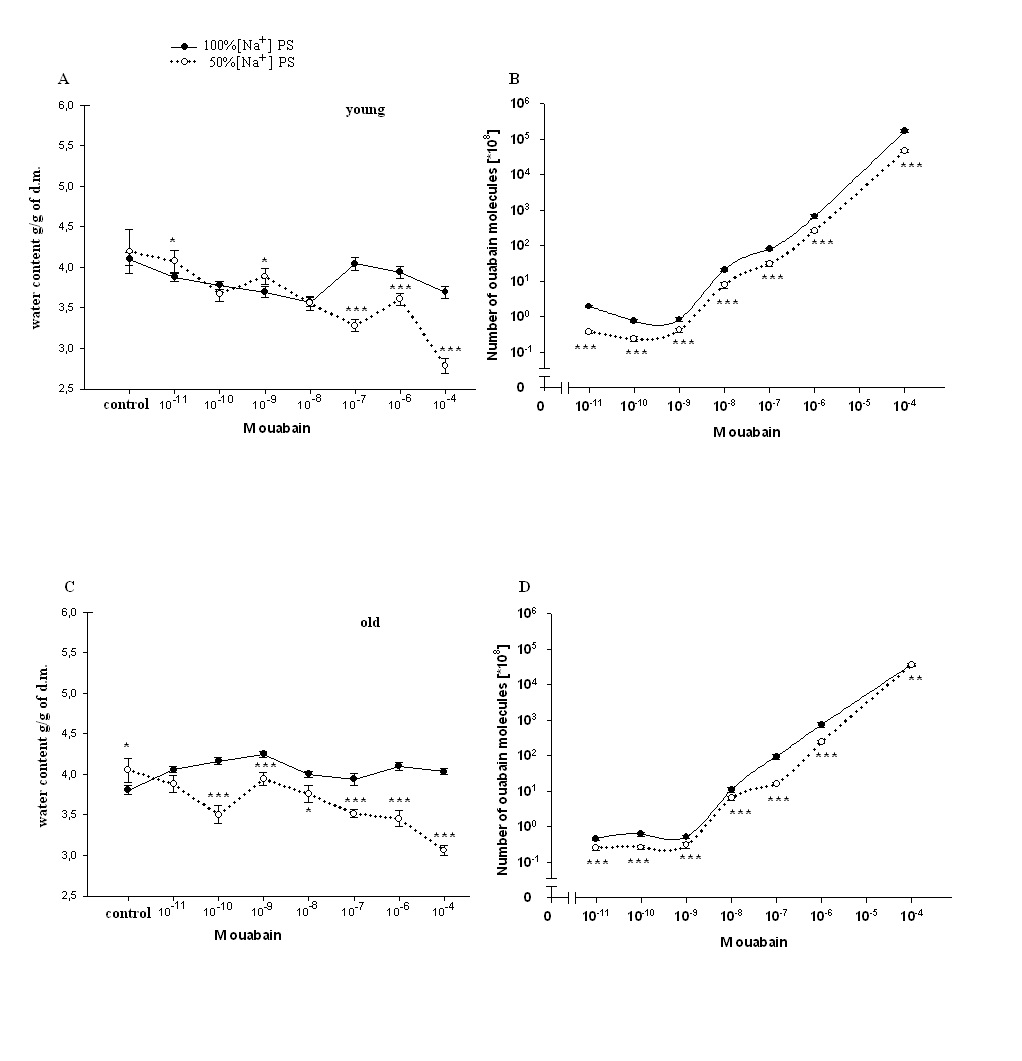
- In Figure 1 dose-dependent binding of ouabain with cell membrane (A) and dose-dependent muscle tissue hydration (B) in young and old rats are presented. As can be seen on the presented curves, in heart muscle tissues of young rats α3-(10-11-10-9M); α2-(10-9-10-7M) and α1- (10-7-10-4M) can be clearly distinguished, while, in heart muscle tissues of old rats these components were less expressed. Dose-dependent [3H]-ouabain binding with α3 receptors had downregulation character which was more pronounced in heart muscle tissues of young animals that in old ones (Figure 1A).
4. Discussion
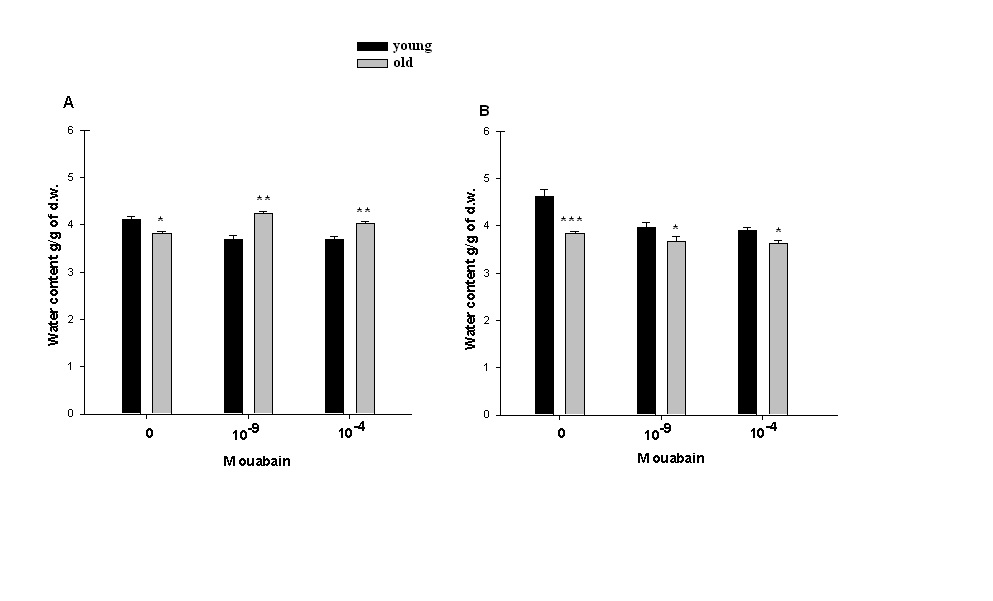
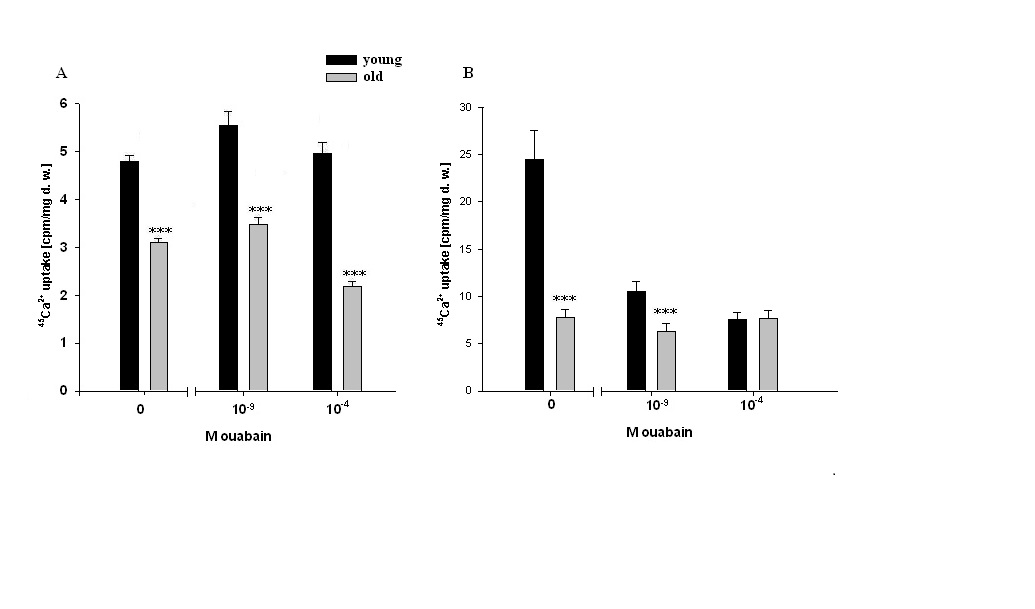
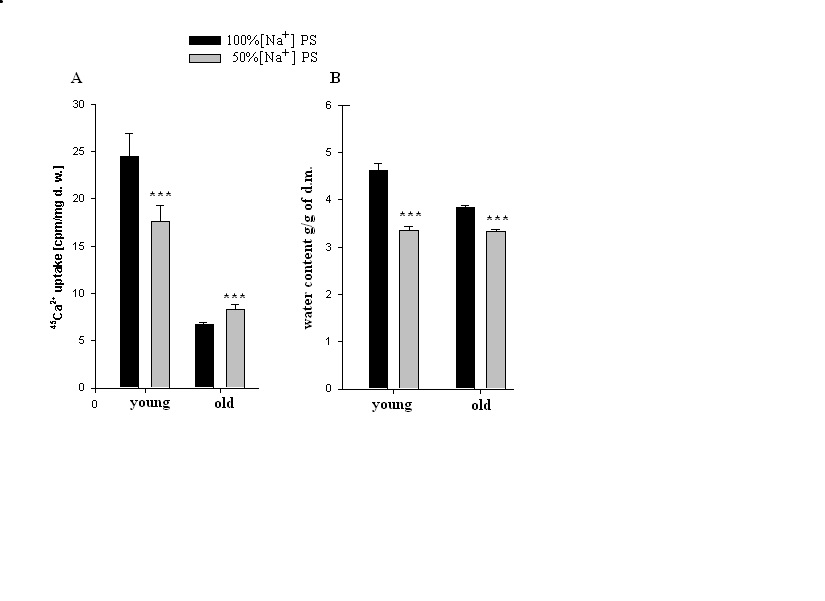
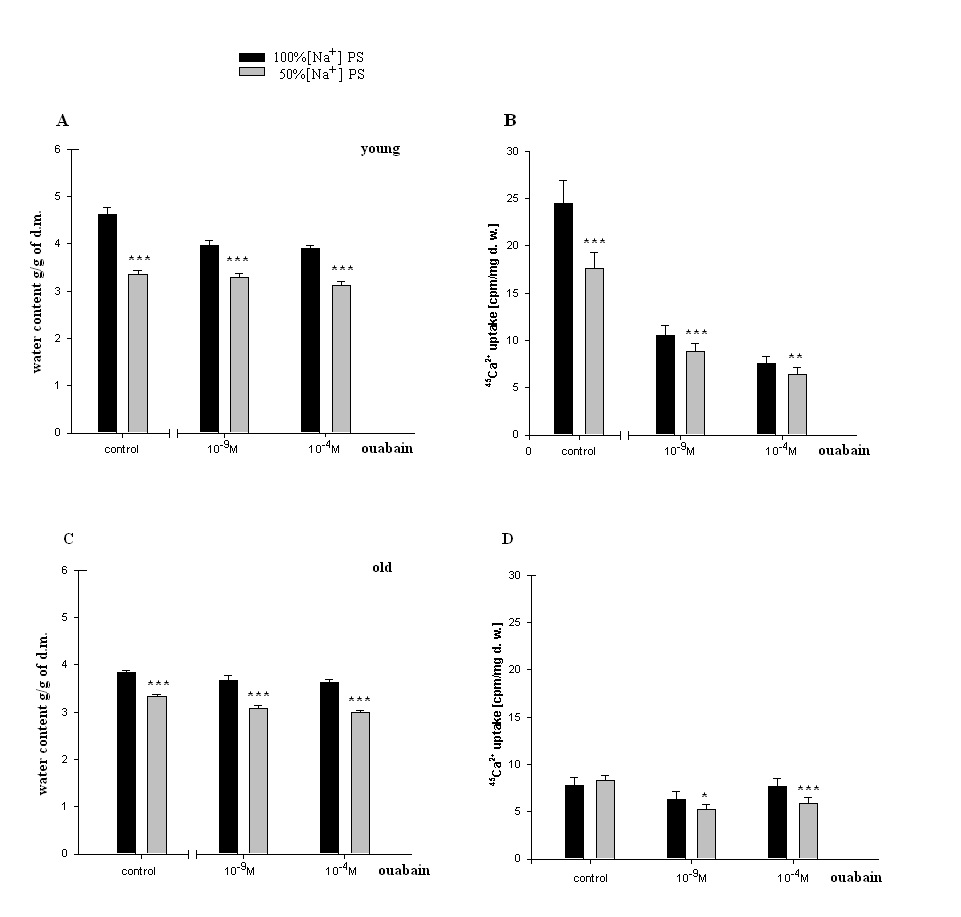
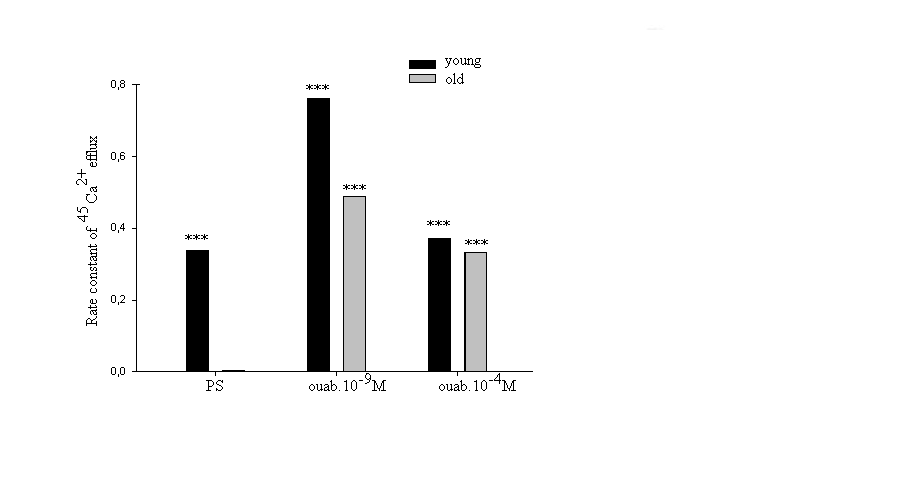
- Approximately 50% of cardiomyocyte volume is made up of myofibrils, and the remainder consists of mitochondria, nucleus, sarcoplasmic reticulum (SR) and the cytosol [48].Therefore, it is obvious that myofibril contractility could have an essential role in regulation of myocyte volume and the latter could be considered as a marker for myocyte contraction.As cell membrane is highly permeable for water, cardiomyocyte volume is controlled by cell metabolic activity, which is realized by two close-talking pathways; by regulation of cytoplasm osmolarity and myofibril contraction.It is known that the inhibition of Na+/K+-pump on one hand leads to the activation of cAMP-Ca2+-dependent phosphorylation processes bringing to myofibril contraction (cell dehydration) and on the other hand to the increase of osmotically active particles in cytoplasm (cell hydration). Previously it has been shown that the ouabain affinity of α3 receptors has more pronounced age-dependent character than α2 and α1 receptors [10]. The obtained data in present work indicate (Figure 1A, B) that <10-9M ouabain-induced activation of α3 in heart muscle of young rats had dehydration effect, while in old rats it had hydration effect. In our previous experiment performed on snail neurons it was shown that <10-9M ouabain had activation effect on 22Na+ efflux in exchange to Ca2+uptake (R Na+/Ca2+ exchange), which was accompanied by elevation of intracellular cAMP, without changing Na+/K+-pump activity [30]. Therefore,<10-9M ouabain-induced dehydration effect on muscle of young rats can be considered as a result of activation of both R Na+/Ca2+ exchange and Ca2+-induced contraction of myofibrils. On the other hand, such factors as NO-donor (SNAP) and magnetic fields, having elevation effect on intracellular cGMP content, have relaxation effects on heart muscle tissues as a result of activation of cGMP-dependent F Na+/Ca2+ exchange. The latter has hydration effect on cells [49]. However, the question of whether nM ouabain-induced muscle dehydration in young and hydration in old rats is due to the activation of R Na+/Ca2+ exchange and F Na+/Ca2+ exchange or contraction and relaxation of myofibrils, respectively, requires further elucidation.Traditionally, ouabain effect on cells is explained by the fact that it has inactivation effect on Na+/K+-ATPase [50]. However, the fact that in vivo experiments 10-9M ouabain had more pronounced dehydration effect on muscle tissues in young and hydration effect in old animals than 10-4M ouabain indicates that the above mentioned explanation cannot be considered reliable.In vitro experiments, where metabolic activity of heart muscle was inhibited (muscle slices bathing in cold K+-free PS), the increase of initial level of muscle hydration in young animals compared with those in vivo experiments can be explained by excitation-induced ions uptake during preparation. In vitro experiments the insensitivity of muscle hydration to depression of cell metabolic activity in old animals can be explained by high initial level of [Ca2+]i-induced muscle contraction (Figure 2A, B). The fact that 10-4M ouabain–induced Na+/K+-pump inhibition had dehydration effect on muscle tissues of young animals (having low [Ca2+]i), and hydration effect on muscle tissues (having high [Ca2+]i) of old animals indicates that ouabain, besides inhibition of transporting function of Na+/K+-ATPase, also has effect on intracellular signaling function leading to muscle contraction in young and hydration (release of endogenous H2O) in old animals, which was depressed in vivo experiments. This suggestion is confirmed by the data on the effect of 10-9M ouabain (having no effect on Na+/K+-pump) on muscle hydration in vitro experiments (Figure 2B). It is worth to note that in vivo experiments 10-9M ouabain-induced muscle hydration in old animals was higher than 10-4M ouabain-induced hydration, which allows us to consider that there are different mechanisms which are responsible for the effects of low and high ouabain concentrations on myocyte hydration. There are minimum two intracellular signaling systems which could be responsible for 10-9M ouabain-induced changes of myocyte hydration in young rats: a) cAMP and Ca2+-dependent myofibril contraction (dehydration) and b) release of endogenous H2O in cytoplasm (hydration) as a result of cAMP-dependent activation of Ca2+-ATPase in ER membrane.In muscle tissues of old animals, where myocytes are in contracted state because of high [Ca2+]i, 10-9M ouabain-induced hydration of myocytes can be explained by activation of Ca2+ efflux from cytoplasm leading to reactivation of membrane ATP-ases and release of endogenous H2O. On the basis of our previous and literature data, we hypothesize that 10-9M ouabain-induced stimulation of Ca2+-calmodulin-NO-GMP-F Na+/Ca2+ exchange cascade could be responsible for myocyte hydration in old animals.Previously we have shown that there is a negative correlation between Na+/K+ATP-ase and cAMP formation (adenylatecyclase activity) [51]. This correlation is disturbed by the increase of phospholipase A2 activity [52-53]. It is known that the increase of [Ca2+]i leads to the activation of phospholipase activity in membrane [54]. Therefore, it is suggested that in old animals inositol triphosphate (I3P) leading to activation of phosphatidylinositol cycle producing cGMP could serve as a sensor for 10-9M ouabain and activate Na+/Ca2+exchange in ER [46] and cell membrane [54]. This conclusion cannot be final and needs more detailed investigation.The data that in vivo experiments 45Ca2+ uptake by heart muscle tissues of young animals was significantly higher than in old ones can be explained by age-dependent increase of [Ca2+]i. Traditionally, cardiac glycoside-induced stimulation of heart muscle contractility is explained by Na+/K+-pump inhibition-induced increase of [Ca2+]i through the activation of R Na+/Ca2+ exchange in cell membrane [26,55]. As Na+/K+-pump has age-dependent dysfunctional character [56], it was predicted that pump inactivation-induced stimulation of R Na+/Ca2+ exchange could be more expressed in heart muscle tissues of young animals than in old ones. However, the obtained data have shown that poisoning by10-4M ouabain led to the depression of 45Ca2+ uptake by muscle tissues of old animals, while it had slight activation effect on 45Ca2+ uptake in muscle tissues of young ones (Figure 3A). These data witness the absence of negative correlation between Na+/K+-pump and R Na+/Ca2+exchange in heart muscle, which was present in intracellularly perfused squid axon [55]. The obtained data speak about the existence of metabolic mechanisms controlling 45Ca2+ absorption properties of intracellular structure, such as ER and mitochondria, which have age-dependent dysfunctional character. The data obtained in vitro experiments (Figure 3B), where 10-4M ouabain had strong depressing effect on45Ca2+ uptake in both young and old animals and this effect had no age-dependent character, clearly indicate that 10-4M ouabain-induced changes of 45Ca2+ uptake cannot be explained only by inhibition of ion transporting function of Na+/K+-pump.The fact that intracellular signaling system controlling 45Ca2+ absorption properties of intracellular structure has a crucial role in regulation of 45Ca2+ uptake by heart muscle tissues is supported by the data presented in Figure 3A, B. 10-9M ouabain had more pronounced activation effect on 45Ca2+ uptake by muscle tissues of young and old animals than 10-4M ouabain in vivo experiments. These data witness the existence of different mechanisms responsible for low and high concentration effects of ouabain on 45Ca2+ uptake by muscle tissues. Furthermore, the reverse effect (inhibition) of 10-9M ouabain on 45Ca2+ uptake in vitro experiments compared with the data obtained in vivo experiments indicates the metabolism-dependent character of this effect (Figure 3B).This suggestion is confirmed by the data of the comparative study of dose-dependent ouabain effects on R Na+/Ca2+ exchange and hydration in muscle tissues bathing in 100% and 50% [Na+] PS.It is known that the decrease of [Na+]o brings to the activation of R Na+/Ca2 exchange [55]. However, the obtained data indicate that 45Ca2+ uptake was decreased in young animals upon the effect of 50% [Na+] PS, whereas, in old animals 50% [Na+] PS led to the increase of 45Ca2+ uptake (Figure 4A). Although 50% [Na+] PS had depressing effect on 45Ca2+ uptake by heart muscle tissues in young and activation effect in old animals, it had dehydration effect on heart muscle tissues in both ages of animals (Fig. 4B). Furthermore, dehydration effect of 50% [Na+] PS on muscle tissues of young animals was more pronounced than in old ones. Therefore, it is suggested that 50% [Na+] PS-induced muscle dehydration cannot be explained by R Na+/Ca2 exchange, but by [Ca2+]i-induced myosin contraction, which had age-dependent weakening character (Figure 4B). The data on the study of ouabain effects on 45Ca2+ uptake at 50% [Na+] PS in vitro experiments bring us to the same conclusion.The data that both low and high concentrations of ouabain (10-4M and 10-9Mouabain) had depressing effect on 45Ca2+ uptake, but had no effect on muscle tissue hydration clearly indicate that in vitro condition, when muscle tissues were enriched by [Ca2+]i, myocytes were in contracted state and were insensitive to the changes of [Ca2+]i (Figure 5). Furthermore, 10-9M ouabain in young animals had less inactivation effect on 45Ca2+ uptake than 10-4M ouabain, while in old animals it had more inhibitory effect on 45Ca2+ uptake than 10-4M ouabain. These data allow us to suggest that 10-9M ouabain effect depends on the initial level of [Ca2+]i. The data indicating that dose-dependent ouabain binding with cell membrane in muscle tissues of both young and old animals was depressed at 50% [Na+] PS compared with 100% [Na+] PS (Figure 6B,D) can be explained by the increase of [Ca2+]i..It is suggested that the increase of [Ca2+]i can bring to the decrease of ouabain binding with membrane by two mechanisms; a) by myosin contraction leading to the decrease of the number of ouabain receptors on cell surface b) by the decrease of affinity of membrane receptors to ouabain. At the range of <10-9M ouabain concentrations (α3 receptors), 50% [Na+] PS-induced decrease of ouabain binding in young animals was accompanied by the increase of muscle hydration (Figure 6B) (except at 10-10M ouabain). This allows us to exclude myosin contraction-induced decrease of the number of α3 receptors in membrane and consider this depression as a result of the decrease of ouabain receptors affinity. Therefore, the data indicating that ouabain binding with α3 receptors in young animals is more pronounced (Figure 6A) that in old ones (Figure 6C), allow us to explain it by high initial level of [Ca2+]i in old animals. These explanations are in harmony with literature data indicating that the affinity of α3 receptors to Ca2+ is higher than of α2 and α1 receptors [57].The data showing that all concentrations (10-11-10-4M) of ouabain had dehydration effect on heart muscle tissues of old animals (Figure 6D), while <10-9M ouabain had hydration effect on heart muscle tissues of young animals allow us to suggest the existence of an age-dependent signaling system, through which 50% [Na+] PS leads to formation of endogenous H2O. Thus, on the basis of the above presented data it can be concluded that the activation of R Na+/Ca2+ exchange in young animals was accompanied by the stimulation of intracellular signaling system leading to the release of endogenous H2O which was depressed in old animals (Figure 6B,D). This suggestion is supported by the data of in vitro experiments on age-dependent study of the effects of 10-9 M and10-4 M ouabain on the rate of 45Ca2+ efflux from heart muscle tissues (Figure 7).[Ca2+]i can be decreased by removing Ca2+ from the cytosol by various mechanisms; a) by Ca2+-pumps in membrane of endoplasmatic reticulum (ER) and in PM pushing Ca2+ into ER or outside the cell, respectively, b) by Na+/Ca2+ exchange in forward mode (F Na+/Ca2+ exchange) extruding cytosolic Ca2+ in exchange for extracellular Na+. Ca2+-pump in cell membrane controls the rest Ca2+ concentrations because of their high-affinity/low-capacity transport properties, whereas, Na+/Ca2+ exchangers display low-affinity/high-capacity transport properties [21]. Therefore, in case of high [Ca2+]i, when Ca2+-pumps in ER and PM are in inhibited state, the net Ca2+ efflux is mainly determined by F Na+/Ca2+ exchange [55].The fact that the initial rate of 45Ca2+ efflux from the muscle tissues of young animals was much higher than of old ones (Figure 7) can be considered as a determining factor for age-dependent weakening character of 45Ca2+ uptake intensity (Figure 3). The fact that 10-9M ouabain had stronger stimulation effect on the rate of 45Ca2+ efflux from the muscle tissues of young animals than of old ones allows us to consider age-dependent weakening of nM ouabain-induced activation effect of 45Ca2+ uptake as a result of activation of 45Ca2+ efflux from the myocytes (Figure 3A). Both in young and old animals 10-9M ouabain had stronger activation effect on 45Ca2+ efflux than 10-4M ouabain (Figure 7). Moreover, the activation effect of 10-9M ouabain on the rate of 45Ca2+ efflux had age-dependent weakening, while 10-4M ouabain had age-dependent elevation character. These data serve as strong evidence that different metabolic mechanisms are responsible for the activation effect of 10-9M ouabain and 10-4M ouabain on the rate of 45Ca2+ efflux. Thus, the obtained data of the present work allow us to make the following conclusions:1. In young animals muscle hydration is significantly higher than in old ones.2. In heart muscle of young animals dose-dependent ouabain binding with cell membrane in the range of 10-11-10-9M has downregulation, while in old ones it has upregulation character. These concentrations of ouabain have hydration and dehydration effects on heart muscles of young and old rats, correspondingly. 3. In vivo experiments the intensity of 45Ca2+ uptake by heart muscle of young animals is significantly higher than in old ones, while in vitro experiments such age-dependency of 45Ca2+ uptake intensity is eliminated.4. In vivo experiments 10-9 M ouabain has activation effect on 45Ca2+ uptake by muscles which has age-dependent weakening character, while in vitro experiments it has depression effect on 45Ca2+ uptake which also has age-dependent weakening character. 5. In vitro experiments PS with 50% [Na+] has depression effect on 45Ca2+ uptake by muscles in young and activation effect in old animals.Whereas, in both age groups PS with 50% [Na+] leads to muscle dehydration, which in young animals is more pronounced, than in old ones. 6. The intraperitonal injection of PS with 50% [Na+] has depression effect on [3H]-ouabain binding with membrane with α3 receptors but has different dose-dependent ouabain effect on muscle hydration in the range of 10-11 -10-9 M ouabain in young and old animals. 10-11, 10-9 M ouabain have hydration and 10-10 M ouabain has no effect on heart muscles of young animals. Whereas, in old animals 10-11 -10-9 M ouabain have dehydration effect. 7. The rate of 45Ca2+ efflux from heart muscle has strong age-dependnet depressing character. 10-9,10-4M ouabain have stimulating effect on 45Ca2+ efflux from muscle which has age-dependent weakening character. The activation effect of 10-9 M ouabain is more pronounced on the rate of 45Ca2+ efflux than the activation effect of 10-4 M ouabain. 8. The data that ≤10-9 M ouabain has stronger effect on both muscle hydration and 45Ca2+ exchange than 10-4 M ouabain, indicate that the effects of ≤10-9 M ouabain on muscle hydration and 45Ca2+ uptake cannot be explained by inactivation of Na+/K+ pump which takes place in case of 10-4 M ouabain.9. Based on the previous data that ≤10-9 M ouabain has activation effect on cAMP-dependent R Na+/Ca2+ exchange and the present data that ≤10-9 M ouabain has activation effects on muscle hydration and 45Ca2+ uptake which has age-dependent weakening character and disappears in case of depression of metabolic activity of muscles (in vitro experiments at 7oC) allow us to conclude that age-dependent dehydration of heart muscle is a result of dysfunction of cAMP-dependent Na+/Ca2+ exchange-induced activation of oxidation processes bringing to the release of endogenous water molecules in cytoplasm.
ACKNOWLEDGEMENTS
- We express our gratitude to Ani Gyurjinyan and Anna Sargsyan for editing the article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML