-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(3): 64-72
doi:10.5923/j.als.20150503.03

The Ecology of the Saline Lakes in the Semiarid Pampa Central (Argentina): Limnologic Characterization and Zooplankton of Utracán
Echaniz Santiago, Cabrera Gabriela, Vignatti Alicia
Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, La Pampa, República Argentina
Correspondence to: Echaniz Santiago, Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, La Pampa, República Argentina.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

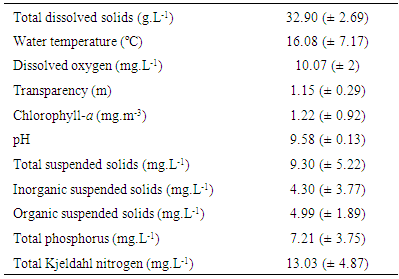
In the semi-arid central Argentina exist temporary saline lakes that have recently begun to be studied. If salinity does not exceed 30 g.L-1 can be studied using the “alternative states of shallow lakes model”. The aim of this study was to determine the ecology of a limnologically unknown lake during a mesosaline period and to verify that due to the salinity, zooplankton richness, density and biomass were similar to other lakes in the region and, due to the fishes’ lacking, the zooplankton is consisted of larger species, which contributes to the clear state. The depth and salinity remained stable (2 ± 0.15 m and 32.9±2.7g.L-1). Although high concentrations of nutrients, transparency exceeded 1.1 m and chlorophyll-a concentration was around 1mg.m-3. The ratio Zm / Zphot was 0.62 (clear state). Two cladocerans, two copepods and two rotifers were registered and no correlation between richness and environmental parameters was found. The greatest density and biomass was provided by copepods, especially Boeckella poopoensis Marsh, 1906. The zooplankton biomass was lower than other Pampean lakes, probably by the limited availability of food derived of the clear state. A particularity of the lake was the reduced influence of environmental factors on zooplankton, probably due to varied in narrow ranges and the species remained within its tolerances ranges.
Keywords: Saline lakes, Clear state, Zooplankton, Boeckella poopoensis
Cite this paper: Echaniz Santiago, Cabrera Gabriela, Vignatti Alicia, The Ecology of the Saline Lakes in the Semiarid Pampa Central (Argentina): Limnologic Characterization and Zooplankton of Utracán, Advances in Life Sciences, Vol. 5 No. 3, 2015, pp. 64-72. doi: 10.5923/j.als.20150503.03.
Article Outline
1. Introduction
- The province of La Pampa is located in the central semiarid of Argentina. It is in a region where the mean annual rainfall show a steep gradient, with 700 mm to the east to less than 300 mm to the west [1], but they are always exceeded by evapotranspiration, which is around 800 to 850 mm per year in the whole region [2]. In this area there are numerous shallow lakes located in arheic basins, primarily fed by rainfall and to a lesser extent by groundwater contributions. Due to the high evaporation of the region, one of the main characteristics of these lakes is the temporality, which makes that containing water for a few weeks or a few years, after which it can dry completely, also for several years. Most of these lakes have concentrations of dissolved solids higher than 3 g.L-1, so they can be classified as saline lakes [3-6], but the dynamics given by the filling-drying make their salinity widely vary [7-10].It is known that salinity is one of the abiotic factors that mainly influences the biota of these lakes [5, 11, 12], since both zooplankton richness and density are decreasing with increasing the concentration of dissolved solids [3, 13-18]. This phenomenon has been recently verified in La Pampa [5, 6, 12].While the salinity of these lakes does not exceed about 30 g.L-1 and preventing the presence of large size cladocerans, the theoretical framework that has proved to be suitable for study is the alternative states of shallow lakes model [19, 20, 21]. This indicates that the presence of planktivorous fishes in an aquatic ecosystem produces an effect of trophic cascade since the predation exerted by fishes on larger size zooplankton species with greater filtration efficiency, leads to increased phytoplankton chlorophyll-a concentration and a decrease in water transparency (turbid state) [19, 21-24]. On the other hand, the absence of planktivorous fishes (or low density) favours the development of large size zooplankton cladocerans, especially of the genus Daphnia, which due to its high rate of grazing, maintain low concentrations of chlorophyll-a and, consequently, the transparency of the water is high (clear state) [19, 21-24]. This model has been tested in some relatively saline lakes of La Pampa, where the presence of the halophilic Daphnia menucoensis Paggi, 1996 determines clear states [8, 12, 25].The limnological characteristics of saline, shallow and temporary lakes elsewhere are known, but in Argentina this type of ecosystems have not received much attention, except for some relatively stable saline lakes in the provinces of Buenos Aires [26-28] and Santa Fe [29]; highland saline lakes in the northwest of the country [30, 31], the Mar Chiquita lake, in Córdoba province [32]. In the central semiarid region of Argentina this kind of lakes is abundant but only recently has begun the study of some of them, pointing out a high variation in their physicochemical and biological characteristics [9, 10]. Because of that, the aim of this study is to describe the variation of the physical, chemical and biological parameters (taxonomic composition, richness, density and zooplankton biomass) and to establish the relationships between them, into a shallow saline lake in the province of La Pampa limnologically unknown, during a period in which the salinity remained within the range mesosaline [3]. In addition to them, another scope of this study is the verification of the following hypotheses: i) due to environmental stress caused by high salinity, zooplankton species richness is reduced, with the presence of halotolerant species; ii) the zooplankton richness, density and biomass are similar to the other lakes of La Pampa having the same range of salinity and iii) due to temporality and high salinity, the lake lacks predatory fishes, so the zooplankton community is mostly composed of large size species, with cladocerans that determine the lake have reduced concentration of phytoplankton chlorophyll-a and consequently, high water transparency (clear state).
2. Materials and Methods
2.1. Study Area
- The Utracán Lake (64°36´W, 37°17´S) (Figure 1) is temporary, of an elongate shape, and follows the direction of a depression in a valley of the same name. During the study it had a maximum length and width of 2333 and 649 m, respectively, an area of 96.6 ha and a maximum depth of 2.2 m. It is located in the phytogeographic province of the Thorny Forest [33], surrounded by natural vegetation, with grasslands of Hyalis argentea D. Don. Ex Hook. & Arn. and Stipa brachychaeta Godron and native forest of Prosopis caldenia Burkart and Geoffroea decorticans (Gill. Ex Hook. & Arn.) Burkart.
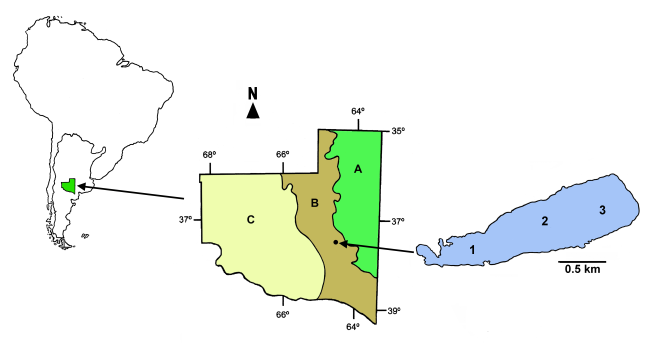 | Figure 1. Geographic location of Utracán Lake. A, B and C: Phytogeographical provinces of the Pampa Plains, Thorny Forest and Monte respectively. 1, 2 and 3: Sampling sites |
2.2. Field and Laboratory Work
- Monthly samples of water and zooplankton were collected in the period January- December 2007 at three sampling sites (Figure 1). The water temperature, the concentration of dissolved oxygen (Lutron® OD5510 oximeter), the water transparency (Secchi disk) and the pH (Corning® PS15 pH meter) were recorded at each station, and water samples, which were kept refrigerated until analysis, were collected for physico-chemical determinations. In addition, a qualitative sample of zooplankton was taken by vertical and horizontal dragging, with a net of 22 cm in diameter at the mouth and mesh opening of 0.04 mm, while quantitative samples were taken with a 10 L Schindler-Patalas plankton trap with a net with a 0.04 mm mesh opening, by means of two vertically aligned extractions, so as to integrate the water column. Samples were anesthetized with CO2 and kept refrigerated until analysis. The maximal depth of the lake was measured by probing and the length and width with a GPS Garmin® E-Trex Legend.The dissolved solids concentration (salinity) was determined by the gravimetric method with drying at 104°C of 50 ml of previously filtered water. The chlorophyll-a concentration was determined by extraction with aqueous acetone to 90% and subsequent reading in a spectrophotometer [36, 37], total Kjeldahl nitrogen (TKN) by the Kjeldahl method and total phosphorus (TP) using the method of ascorbic acid, after previous digestion with potassium persulfate. The content of suspended solids was determined with Microclar FFG047WPH fiberglass filters, dried at 103–105°C until constant weight and later calcined at 550°C [38].Counts of macro and microzooplankton [20] from each sample were carried out under stereoscopic and conventional optical microscopes using Bogorov and Sedgwick-Rafter cameras, respectively. Once analyzed, the samples were fixed with formaline 5% and then deposited in the plankton collection of the Facultad de Ciencias Exactas y Naturales de la Universidad Nacional de La Pampa. To determine the biomass of the zooplankton, a minimum of 30 specimens of all species were measured with a Leitz ocular micrometer and formulas that relate to the total length with the dry weight of the specimens were used [39-43].We used the classification of continental waters based on salinity proposed by Hammer [3]. In order to examine the relationships between environmental factors and attributes of zooplankton, nonparametric Spearman correlation coefficients (rs) were calculated [44-46]. In order to characterize the lake as a function of its transparency, we calculated the relationship between the mean depth and that of the photic zone: Zm/Zphot [47]. If the calculated value is less than 1, it is considered as a clear lake, and if this ratio is greater than 1, it is a turbid one. For the calculation of the depth of the photic zone, we multiplied the reading of the Secchi disk by a factor of 3 [20, 48]. We used Past [49] and Infostat [50] software.
3. Results
3.1. Environmental Parameters
- The depth of the lake was slightly lower when the study began, at around 1.8 m (January and February). It rose in March to about 1.95 m and it remained relatively stable until September, when increased and reached the maximum depth recorded (2.22 m) (Figure 2). The mean dissolved solids concentration was 32.9 g.L-1 (± 2.7); this was also quite stable and followed a reverse pattern with depth, so that a high correlation was found between these two parameters (rs = -0.91; p = 0.0000) (Figure 2).
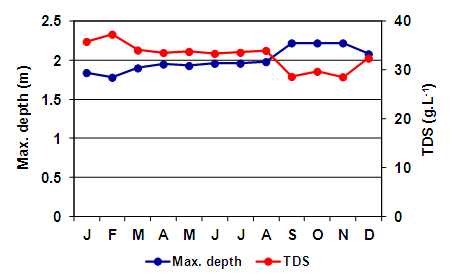 | Figure 2. Monthly variation of the maximum depth and total dissolved solids in Utracán Lake during 2007 |
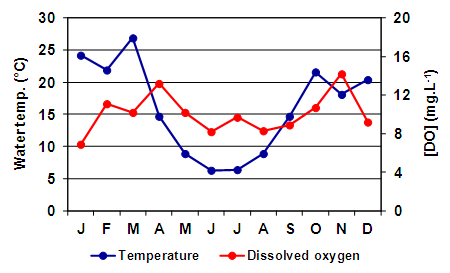 | Figure 3. Monthly variation of the water temperature and dissolved oxygen in Utracán Lake during 2007 |
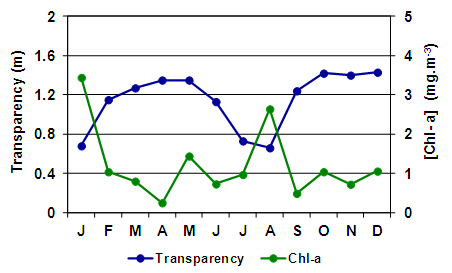 | Figure 4. Monthly variation of the water transparency and chlorophyll-a concentration in Utracán Lake during 2007 |
|
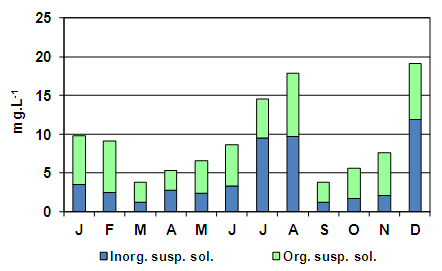 | Figure 5. Monthly variation of the suspended solids concentration in Utracán Lake during 2007 |
3.2. Zooplankton
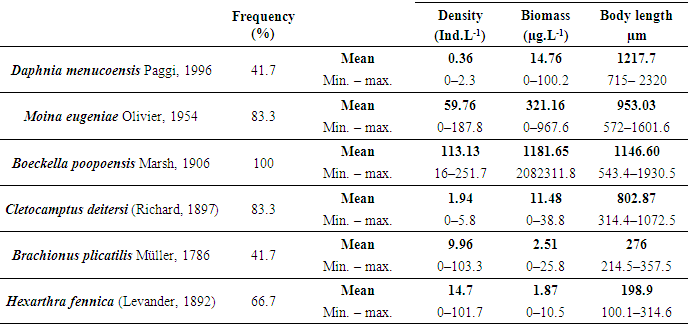
- Six taxa were recorded: two cladocerans, two copepods and two rotifers (Table 2). The greatest richness was recorded in mid-fall (May) when all the six species were found. The lowest richness was recorded during the coldest months (June, July and August) when only three species of crustaceans and no rotifers were recorded. However, no correlation was found between total richness and the water temperature or salinity. Considering these separately, a significant correlation was found between the richness of rotifers and water temperature (rs = 0.59; p = 0.0419) because they were only recorded during the months with higher temperatures.
|
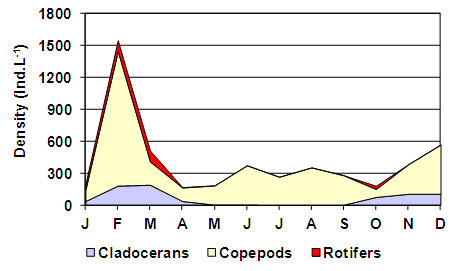 | Figure 6. Variation in the composition of the zooplankton density of the Utracán Lake during 2007 |
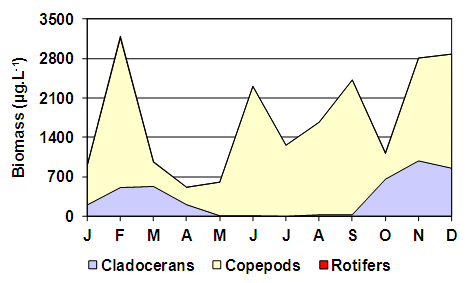 | Figure 7. Variation in the composition of the zooplankton biomass of the Utracán Lake during 2007 |
3.3. Density, Biomass and Size of the Main Species
- Among cladocerans, M. eugeniae was recorded in ten samples. It presented a maximum density of 187.8 ind.L-1 in late summer (March), but as the specimens were of a small size (mean = 784.64 µm ± 173.48) its biomass was not so high (Figure 8). The density and the biomass of this cladoceran decreased in late fall and early winter and it was not recorded in August and September. It reappeared in mid-spring, in October, after which the largest biomass reached a peak of 967.6 μg.L-1 in November, although as larger specimens were found (mean = 1107.33 µm ± 247.89), the density was not as high as in summer (Figure 8). The mean size of M. eugeniae (Table 2) showed a significant correlation with salinity (rs = -0.74; p = 0.0365). Daphnia menucoensis Paggi, 1996 was recorded only five times and its density was reduced (Table 2). Except for an occasional record in May, it was only found from early spring. It reached the greatest density and biomass in October, with 2.33 ind.L-1 and 100.2 µg.L-1, respectively. Correlations between the mean size of this species (Table 2) and the concentration of total suspended solids were found, particularly with those of organic origin (rs = -0.90; p = 0.0374).
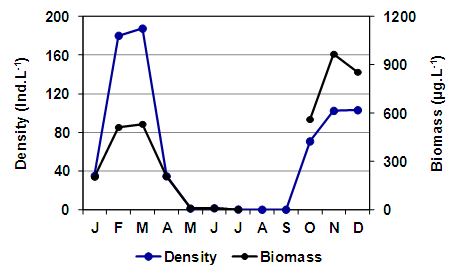 | Figure 8. Changes in the density and biomass of Moina eugeniae in the Utracán Lake during 2007 |
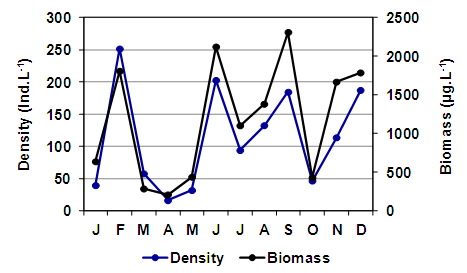 | Figure 9. Changes in the density and biomass of Boeckella poopoensis in the Utracán Lake during 2007 |
4. Discussion
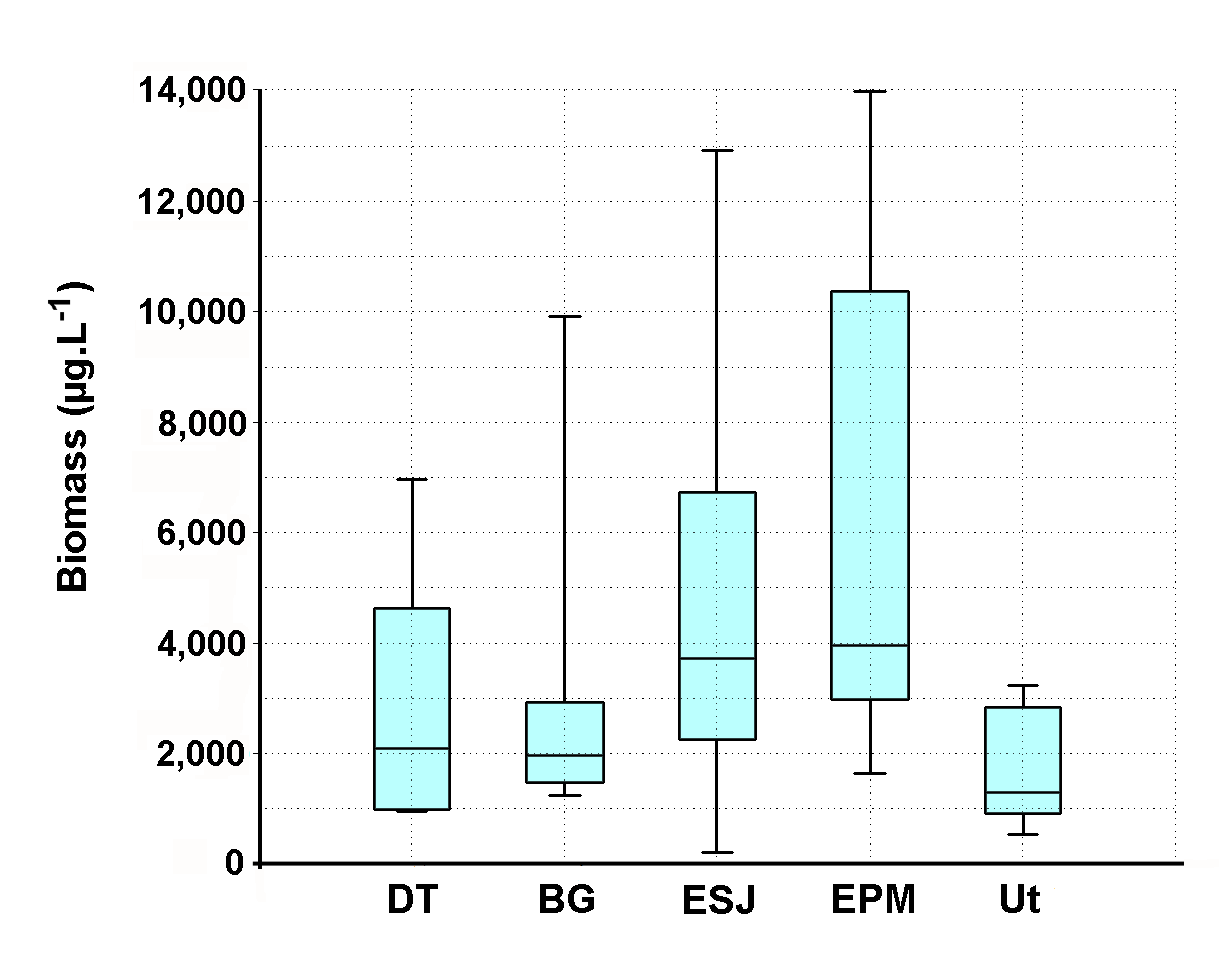
- Despite the temporary nature of Utracán – it was revealed both before and after 2007 that the lake level showed significant declines (Echaniz & Vignatti, pers. obs.) – during the studied period its depth and its salinity remained relatively stable. The oscillations barely reached 0.4 m and 9 g.L-1, which kept it in the mesosaline range [3], while during the same period other Pampean lakes recorded declines in water level above 25% and salinity increases reached double the initial values [10]. The chemical composition of the water, with the predominance of Na+ between cations and the Cl- between anions, is a characteristic similar to most of the shallow lakes in the province and indicates that the mechanisms predominate in controlling water chemistry involving evaporation and crystallization. This is a typical situation in arid or semiarid regions such as the central region of La Pampa, where evapotranspiration rates are higher than rainfall [20, 51, 52].The low value of the Zm/Zphot ratio found allows Utracán to be characterized as a clear lake and the phytoplankton chlorophyll-a concentrations and suspended solids were reduced, which differs from other shallow lakes of La Pampa, which are strongly turbid. In many, the reduced transparency is due to high concentrations of phytoplankton chlorophyll, a situation promoted by the absence of large-sized zooplankton because of predation by zooplanktivorous fishes [25, 53, 54], and in others lakes to high concentrations of inorganic suspended solids in the water column [10, 55, 56]. The high transparency of the water of Utracán may be the result of, on the one hand, the presence of cladocerans of a relatively large size and therefore efficient filtering, due to the absence of predation by fishes, which cannot colonize this ecosystem because of its saline and temporary nature. On the other hand, the transparency allowed light to reach the bottom, which led to the development of R. cirrhosa. The fact of having a large proportion of the bed covered by the macrophyte has contributed to the fact that the sediments were not resuspended by wind. However, the minimum transparency, produced by inorganic suspended solids, was recorded in August, the month with a historical record of strongest winds in the region [57]. The concentrations of nutrients measured were so high that it allowed Utracán to be categorized as a hypertrophic lake [58]; however, they were similar to other environments in La Pampa [6, 59, 60, 61]. This could well attributed in part to the drag, especially during storms, of the excreta of animals that graze in the basin [56, 62, 63, 64], and partly to the resolubilization of nutrients from the sediments [65, 66]. The latter is a particularly important phenomenon in shallow lakes [67-69], and in Utracán it coincided with the highest concentrations of nutrients in the water being recorded during July and August, the months with the strongest winds [57]. In La Pampa it has been found that subsaline lakes not exceeding 2 g.L-1 may have more than 20 taxa in their zooplankton [4, 54, 59, 61], while in hypo-mesosaline lakes, where salinity has a major influence in the structuring of their community [3, 11, 18], the richness is lower [60]. The latter situation was verified in Utracán, where the diversity of zooplankton was reduced and the association is characterized particularly by halophilic native species, such as D. menucoensis, M. eugeniae and B. poopoensis [6, 59, 60, 70]. The two first species are very common in saline ecosystems of northern Patagonia and the central region of the country [10, 12, 59, 60, 71], while B. poopoensis shows a much wider geographical distribution, from the north of the Patagonian plateau to the south of Peru [30, 72, 73]. Among rotifers, the presence of eurihaline species such as B. plicatilis and H. fennica is a commonly characteristic with other similar water bodies in the province [12, 59, 60, 74], although there are species that are not typical of the region due their cosmopolitan distribution [75]. On the other hand, the prevalence of crustaceans, especially with the high density of B. poopoensis, and the limited participation of rotifers are a characteristic point which distinguishes Utracán from other saline lakes without fishes, where the highest density was provided by species of the genus Brachionus, particularly B. plicatilis, B. dimidiatus and B. angularis [8, 9, 10].The zooplankton biomass recorded in Utracán was much lower than that recorded currently in other shallow lakes in La Pampa (Figure 10). It was almost the half that recorded in Don Tomás, a subsaline lake adjacent to the city of Santa Rosa, or 40% lower than that found in the hyposaline Bajo de Giuliani. This occurred despite the fact that in both lakes fish fauna with a predominance of zooplanktivorous fishes was recorded, which suggests that most of the zooplankton species were smaller but they reached very high densities, with a predominance of cyclopoid copepods and rotifers [54, 76]. The Utracán biomass was also between 2.7 and 3.7 times less than that of the lakes located in San Jose and Pey-Ma, respectively [9, 10]. This could be attributed to the fact that, although they are also mesosaline lakes and lack fishes like Utracán, during the annual cycles studied phytoplankton chlorophyll-a concentrations were between 20 and 30 times higher, and that of suspended solids 3 to 7 times higher, which could suggest a much larger supply of food for zooplankton. In Utracán, it is likely that the shadowing effect caused by the presence of R. chirrosa may have limited the development of phytoplankton, and thus the amount of food available, which may have prevented as high a development of biomass as in the San Jose and Pey-Ma lakes. However, one point in common among these ecosystems is the predominance of calanoid copepods, especially B. poopoensis, since in all three lakes this was the species that mostly contributed to the biomass.
5. Conclusions
- In La Pampa province (central Argentina) exist many temporary saline lakes whose ecology is poorly known, among them is Utracán Lake. The study of the lake during a mesosaline period showed that the zooplankton richness was very low, with the predominance of the calanoid Boeckella poopoensis Marsh, 1906. Two particularities of Utracán were the reduced influence of environmental factors on zooplankton, probably because they remained relatively stable and the species were always within their tolerances ranges and that zooplankton biomass was lower than other Pampean lakes, probably by the scarce availability of food.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML