-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(3): 58-63
doi:10.5923/j.als.20150503.02
Comparative Analysis of Microbial Load of Commercially Prepared and Traditionally Homemade Yoghurt (Ergo) Retailed in Addis Ababa
Kidist Fikre Worku 1, Anteneh Tesfaye Tefera 2, Fassil Assefa Tuji 3
1School of Nutrition, Food Science and Technology, Hawassa University, College of Agriculture, Ethiopia
2Industrial Biotechnology Unit, institute of Biotechnology, Addis Ababa University, Ethiopia
3Department of Microbial, Cellular and Molecular Biology, Addis Ababa University, Ethiopia
Correspondence to: Kidist Fikre Worku , School of Nutrition, Food Science and Technology, Hawassa University, College of Agriculture, Ethiopia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The microbial load and type, acid titration, moisture and microbial frequency of forty yoghurt samples collected from two manufacturing companies (Sholla Dairy Farm and Mama Dairy Farm) and homemade Ergo were analyzed in this study. The mean pH value of the samples varies between 4.07 to 3.5. Moisture content and titration of homemade Ergo samples were above 97% and around 3 ml/l, and for commercially manufactured yoghurt were 82% and above 3 ml/l, respectively. The microbial load analyses of homemade Ergo indicated that lactic acid bacteria, aerobic mesophilic bacteria and psychrotrophic bacteria counts were above log 9 cfu/ml; whereas it was log 7 cfu/ml for commercial samples except for psychrotrophic bacteria which was less than log 5 cfu/ml. Counts of coliforms, fecal coliforms were around log 6 cfu/ml in homemade yoghurt; whereas they were not detected in both commercial samples (Mama and Sholla). In both commercially produced samples yeast, molds, Staphylococcus aureus counts were less than log 5cfu/ml. The counts of Salmonella and Shigella in homemade Ergo were below log 1.5 cfu/ml; and both were not detected in the commercial products. The acid titration, moisture and pH values of both commercial produced and homemade yoghurt were almost similar. However, the microbial count of homemade yoghurt was found to be higher than commercially manufactured products. The higher microbial count of mesphilic bacteria together with detection of the coliforms together with foodborne pathogen indicated the safety implication of the homemade Ergo.
Keywords: Homemade Ergo, Foodborne pathogens, Fermentation, Microbial isolates, Coliforms, Yoghurt
Cite this paper: Kidist Fikre Worku , Anteneh Tesfaye Tefera , Fassil Assefa Tuji , Comparative Analysis of Microbial Load of Commercially Prepared and Traditionally Homemade Yoghurt (Ergo) Retailed in Addis Ababa, Advances in Life Sciences, Vol. 5 No. 3, 2015, pp. 58-63. doi: 10.5923/j.als.20150503.02.
Article Outline
1. Introduction
- Yoghurt is a vast food category with a long and rich history. Like other fermented foods, such as wine and cheese, yoghurt was probably discovered by accident, and its exact origins are unknown. However, its early history is likely interwoven with the general history of agriculture (Kosikowski, 1997). It is a fermented product obtained by lactic acid fermentation of milk by the action of lactic acid bacteria (LAB) (Rasic and Kurmann, 1978). Advisory guidelines for microbiological quality have suggested that satisfactory yoghurts should contain more than l08cfu ml-1 of the starter organisms, < 1 coliform cfu/ml, < 1 mold cfu/ml and < 10 yeasts cfu/ml (fruit-containing yoghurts may contain up to 100 yeasts ml-1and remain of satisfactory quality) (Serhan, 1999). Similarly, microbiological quality of yoghurt commercialized in Brazil showed that adequate microbiological quality must ensure low levels of contamination by coliform microorganisms (Rodrigues, et al., 2010). In French Standards, it was indicated that; the total Fecal coliform count must be lower than 100 cfu/ml of Yoghurt, Salmonella should be absent from 25 ml of sample, and the count of Staphylococcus aureus should be not higher than 10 cfu/ml (Libnor, 2004). Indeed, the yeast and mold count must be lower than 103cfu/ml (Libnor, 2006). Ethiopian Standards Agency also accepts this international microbial load count (ES ISO 21187, 2012). Ergo is a traditional Ethiopian fermented milk product, which has some resemblance to yoghurt. It has thick, smooth and uniform appearance. It has white milky color when prepared carefully. It constitutes a primary sour milk product from which other products may be processed (Almaz Gonfa et al., 2001). As the major fermented dairy product, Ergo is popular and is consumed in all parts of the country.Thus far in Ethiopia, there is little information available on microbiological quality comparison of traditionally produced homemade ergo and industrially produced yoghurt. But some related studies were done. Study done on the occurrence and distribution of species of Enterobacteriaceae in Ethiopian traditional dairy products reported the isolation of Entrobacter, Esherichia coli, Klebsiella, Serratia and Salmonella from Ergo sample (Zelalem Yilma et al., 2007). The same study also reported about 20% of Ergo sample might have been contaminated during post processing via cleaning water or handling personnel. Lactococcus, Streptococcus, Leuconostoc and Lactobacillus were shown responsible for the souring process of Ergo (Almaz Gonfa et al., 1999). In the same study it was reported the detection of fairly high numbers of micrococci, coliforms, aerobic mesophilic bacteria and yeast. (Mogessie Ashenafi, 1995) reported from study done on microbial growth during Ergo fermentation from raw milk samples collected from eight dairy farms in Awassa indicated the presence of aerobic mesophilic, coliform counts, lactic acid bacteria and Yeasts. The aim of this study was to assess the safety, microbial load and quality of homemade Ergo and industrially produced yoghurt around Addis Ababa.
2. Materials and Methods
2.1. Sample Collection and Processing
- A total of 40 samples of which 20 samples where homemade Ergo and 20 samples of commercially produced yoghurt (10 Sholla yoghurt, 5 Mama flavored and 5 Mama non-flavored yoghurt) were sampled, transported and analyzed according to standard methods of (Richardson, 1985 and Vanden-Berg, 1988).Microbial enumeration of homemade ergo and industrially prepared yoghurt samplesMicrobial enumeration was determined by spread-plating 0.1 ml of appropriate dilution in duplicates on pre-dried surfaces of different media. For counting Aerobic Mesophilic count (AMB) and Psychotrophic bacteria (PB), Plate Count Agar was used and plates were incubated at 32°C for 48 h and at 7°C for 10 days, respectively. Coliform and fecal coliform were counted using Violet red bile agar (VRBA) plates and the plates were incubated at 37°C and 44°C, respectively. Both coliform and fecal coliform plates were incubated for 24 h. Yeast and molds were counted using Chloroamphenicol Bromophenol Blue (CBB) and plates were incubated at 25°C for 4 days. For counting of Lactic Acid Bacteria (LAB) De man, Rogasa, Sharpe (MRS) (Oxoid) agar was used and plates were incubated anaerobically at 32°C for 48 h. Staphylococcus aureus was enumerated using Mannitol salt Agar and plates were incubated at 37°C for 24 h.
2.2. Identification of Salmonella
- For the detection and enumeration of Salmonella, 25g of each sample was mixed (pre-enriched) with 225 ml buffered peptone water (Oxoid, UK), the pH of the mixture was adjusted to 6-7 and incubated at 37°C for 20 h. From pre-enrichment culture 10 ml was transferred to 250 ml sterile flasks containing 100ml of tetrathionate broth (Oxoid, UK). The broth culture medium was incubated at 43°C for 48 h. Then 0.1 ml of enrichment broth was spread plated in duplicates on Salmonella-Shigella agar (SSA) (Oxoid, UK) and incubated at 37°C for 48h (Genta and Heluane, 2001). Gray or black central spot colonies from SS agar were sub-cultured on MacConkey agar and incubated at 37°C for 24h. The isolates were then tasted gram reaction, catalase test and cellular shape to identify them as salmonella spp (HPA, 2003).
2.3. Identification of Shigella
- For the detection and enumeration of Shigella 25g of each ergo sample was mixed with 225 ml of Shigella enrichment broth. The samples were incubated at 41.5°C under anaerobic conditions (with well closed 500 ml bottle) for 18 h. Then appropriate dilution 0.1 ml was spread plated in duplicates on Hektoen Enteric Agar (HEA). The plates were incubated at 37°C for 24h. The plate were examined for characteristic colonies, which appear green and moist (EN-ISO 21567, 1999).
2.4. Flora Analysis
- After determination of microbial load of the samples, 10 colonies were picked randomly from countable plates of aerobic mesophilic bacteria and purified by repeated plating on plate count agar. The isolates were characterized to genera level using the following tests.
2.4.1. Catalase Test
- A drop of 3% solution of hydrogen peroxide (H2O2) was placed on a clean microscopic slide, and pure colony from 24 hour old culture on plate count agar were transferred and mixed using sterile wire loop. Evolution of gas was considered as a positive test to catalase (Harley, 2002).
2.4.2. Gram Reaction
- Twenty four hours old pure culture colony were picked from plat count agar and put on clean slide and stirred with two drops of 3% KOH for about 2 minutes. The Gram-negative mass was allowed rise with inoculating needle followed the loop to raise 0.5 to 2 cm or more; whereas the gram positive did not show slime (Gregerson, 1978).
2.4.3. Cell Shape and Arrangement
- From plate count agar a single pure colony was picked and transferred and smeared on clean microscope with a drop of sterile water (Wet mount) and covered with cover slip. The preparation was observed under microscope using oil immersion objective. (Harley, 2002).
2.5. Chemical Analysis
2.5.1. Measurement of pH
- The pH of each Ergo/yoghurt sample was determined by blending 25ml Ergo sample in a flask (500 ml) with 225ml distilled water. The pH of the homogenate was then measured using the digital pH-meter.
2.5.2. Titratable Acidity
- From prepared dilution (225ml sterile distilled water with 25 ml Ergo/yoghurt sample) filtration was done with Whatman filter paper. Then titratable acidity was determined by titrating 10g filtered samples with 0.1N NaOH using three drops of phenolphthalein as an indicator. The titration was completed when the purified Ergo/yoghurt sample change its color to pink. Results were expressed in ml of lactic acid per 1000 ml (liter) of sample (Afnor, 1995).
2.5.3. Moisture Content Determination
- The oven drying method was used for determination of the moisture content. Five gram of the samples (Ergo/yoghurt) was weighed in a pre-dried and weighed stainless steel moisture dishes. Then contents were heated in the oven at 105ºC for 4 h, cooled in desiccators and weighed. This was repeated until a constant weight was recorded to calculate the dry weight of the samples (Tarakci, 2003).
2.6. Date Analysis
- The data were statistically analyzed by using variance (ANOVA). Significance was determined at the 95% level; and when coefficient of variance value was greater than 10 it was considered that the tested factor were significant.
3. Results and Discussion
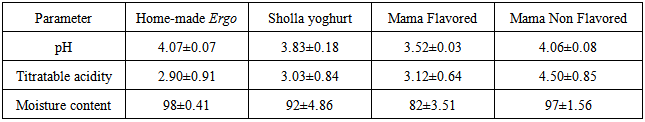
- The mean pH value of homemade Ergo was 4.07.Our result was found comparable with (Zelalem Yilma, 2007) which reported the pH of Ergo samples around 4.02. The mean pH values of Sholla yoghurt and Mama non-flavored yoghurt were found to be 3.83 and 4.06, respectively. Younus et al., (2002) reported that the pH value for market yoghurt was above 4.4, which is higher than results indicated in this study. The mean pH value for Mama flavored yoghurt was 3.52 (Table 1). Moreira et al. (2001) reported a drop of pH by 0.2 units as a result of the addition of the strawberry to fermented milk products. The current result was lower than the pH (4.4) obtained from yoghurt produced from soya beans milk reported by (Akpan, et al., 2007). Variation within the pH values of all samples in this study was not found significant (CV < 10).
|
3.1. Microbial Load of Homemade Ergo Sample
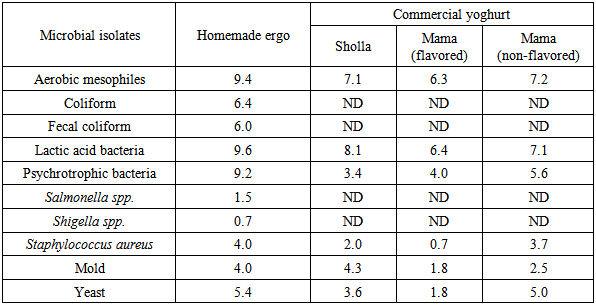
- The average count of LAB was found to be log 9.6 cfu/ml; and that of aerobic mesophilic bacteria (AMB) and psychrophilic bacteria (PB) was shown to be around log 9 cfu/ml. The average count of coliform and fecal coliforms were indicated to be around log 6 cfu/ml. The yeast count of homemade Ergo sample was found to be log 5 cfu/ml, whereas mold and Staphylococcus aureus counts were shown to be ≥ log 4cfu/ml (Fig 1). Count of lactic acid bacteria was very high in homemade Ergo (log 10 cfu/ml) (Fig 1) which were comparable to microbiological characteristics of ghanaian traditional fermented milk product reported by (Akabanda et al. 2010). The counts of Salmonella and Shigella in this study were ≤ log 1.5 cfu/ml (Table 2). Similarly, Salmonella was detected from 4% of Ergo collected in samples collected from different producers Mayssoun and Nadine(2010) reported higher count of Salmonella (log 3 cfu/ml) and lower count of Shigella (log 0.7 cfu/ml) from fermented milk “Laban” in Lebanon. The same authors also showed that such high count may be result from poor hygiene of farms, personnel, utensils. Variations in counts among samples of fecal coliforms, Staphylococcus aureus, yeast and mold were significantly different (CV = 11 – 42). Significant variation in the counts of LAB, AMB, coliforms and PB were not observed (CV < 10), p<0.001.This high microbial load could be associated with luck of insufficient pre-milking udder preparation, insufficient cleaning of milkers’ hands and milking utensils, use of poor quality and non-boiled water for cleaning of udder, transportation of milk and the way it is offered for sale.
|
3.2. Microbial load of Industrially Produced Yogurt Samples
- Sholla yoghurt samplesThe average microbial load analyses of yoghurt from Sholla Dairy Farm of LAB, AMB, yeast/mold, PB and Staph. aureus were indicated to be ≥ log 8 cfu/ml, ≥ log 7 cfu/ml, ≥ log 4 cfu/ml, ≥ log 3 cfu/ml and log 2 cfu/ml, respectively (Fig 2). Salmonella and Shigella were not detected. Similarly neither Salmonella nor Shigella was reported from indigenous milk products by Kumbhar et al. (2009). Except the count of LAB (CV= 8.5) the average counts of other microbial groups were found to vary among samples significantly (CV= 17 – 160) p<0.001.Microbial counts of AMB and LAB from Sholla yoghurt sample were also around log 7 cfu/ml to log 8 cfu/ml (Table 2). In fact high number of aerobic mesophilic counts is not by itself a health risk but it indicates an overall lack of hygiene (Ray, 2004). The microbial load samples from Sholla indicated the dominance of the flora by LAB. Mama flavored samplesThe average microbial load analysis of Mama flavored yoghurt samples exhibited the dominance of LAB and AMB which was shown to be ≥ log 7cfu/ml. The average PB count was shown to be ≥ log 4cfu/ml. Counts of (yeast/ mold) and Staph. aureus were shown to be ≥ log 2 cfu/ml and ≥ log 0.7 cfu/ml, respectively. Salmonella and Shigella were not detected. Significant variations within counts of each type of microorganism among Mama Flavored samples were observed (CV = 57 – 243) p<0.021.Mama flavored yoghurt found to have lower in microbial count than homemade, which could be due to low pH and moisture content. As indicated in Mama flavored Yoghurt sample the pH was less than 4 and contained less bacterial groups than those of homemade Ergo, Sholla Yoghurt and Mama non-flavored Yoghurt which had pH greater than 4 (Mogessie Asshenafi, 2002). Our results were found similar with Nwamaka and chike (2010) and Younus et al., (2002) which reports the counts of total arobic mesophilic bacteria as log 7 cfu/ml for commercially prepared yoghurt. Akabada et al. (2010) and Khan et al. (2008) also reported log 7.4 cfu/ml of aerobic mesophilic count from fermented milk product from Nunu and yoghurt samples collected from Karachi Mega city, Pakistan, respectively. Oyeleke (2009) reported higher aerobic mesophilic counts than the present study (log 8 cfu/ml) from commercially prepared yoghurt retailed in Niger. LAB in industrial product in this study was lower than Rodrigues, et al. (2010) which was reported as log 8 cfu/ml.Mama non-flavored samplesThe average microbial load analysis of Mama non-flavored yoghurt samples showed the dominance of LAB and AMB which was ≥ log 7cfu/ml (Table 2). The mean PB count was shown to be log 5.6 cfu/ml. The average counts of yeast, mold and Staph. aureus were indicated as log 5 cfu/ml, log 2.5 cfu/ml and log 3.7 cfu/ml, respectively. Salmonella and Shigella were not detected. Significant variations in the counts of different groups of microorganisms among samples of Mama non-flavored samples were observed (CV = 11.3 − 123), except the count of AMB (CV = 8.3) p<0.003.Relatively, Mama non-flavored product has high counts of Staphylococcus aureus than Sholla product and Mama flavored yoghurt. Related study also indicated that the process of pasteurization could possibly reduce coagulase-positive Staphylococci significantly but not eliminate them (Bhatt and Bennett 1964).The microbial loads of homemade Ergo samples were found to be higher than the microbial counts of industrially produced yoghurt. This result was found comparable with other studies (Mogessie Ashenafi, 1995) and (Al-Tahiri 2005). The presence of coliforms in industrially produced samples was an indication of unsanitary conditions and/or post pasteurization contamination at one or more stages during processing Abdalla and Abdel Nabi Ahmed (2010). However, coliforms were not detected neither in Sholla yoghurt nor Mama Products. Similar result was also reported for the production and evaluation of yoghurt flavored with solar-dried bush mango (Mbaeyi and Anyanwu 2010).
3.3. Microbial Spectrum of Homemade Ergo and Industrial Yoghurt
- In this study a total of 167 and 121 aerobic mesophilic bacterial colonies were isolated and purified from homemade Ergo and yoghurt samples from dairy farms, respectively.The result showed that 95% and 99% of aerobic mesophilic bacterial colonies from homemade Ergo and industrial yoghurt was shown to be dominated by Gram-positive bacteria, respectively. In-turn, the Gram-positive bacterial flora from homemade Ergo was dominated by Streptococcus spp. (36%) which was followed by Bacillus spp. (23%) and Micrococcus spp. (20%). On the other hand, the Gram-positive bacterial flora from industrial yoghurt was also dominated by Streptococcus spp. (42%) followed by Bacillus spp. (22%) and Micrococcus spp. (21%). Staphylococcus was also encountered from both homemade (16%) and industrial yoghurt (14%) products (Table 3).
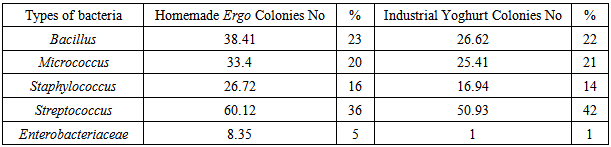 | Table 3. Frequency distribution (%) of the different taxonomic group from Aerobic mesophilic bacteria from homemade ergo and industrial yoghurt purchased from different sampling site in Addis Ababa |
4. Conclusions
- The analyses of homemade Ergo samples showed higher microbial count than industrial products. This could be due to lack of hygiene and sanitary handling during preparation of homemade Ergo, and the presence of high moisture content in the same product. Further analyses of Aerobic mesophilic bacterial flora of Ergo/yoghurt samples showed the dominance of Gram-positive organisms as in the order of Streptococcus followed by Bacillus, Micrococcus and Staphylococcus.
ACKNOWLEDGMENTS
- The authors would like to thank the organizations that are willing to participate in this study. Moreover, we would like to acknowledge the Department of Cellular, microbial and Molecular biology of Addis Ababa University for facilitating laboratory space and provision of basic facilities. Addis Ababa University financed this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML