-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(2): 48-52
doi:10.5923/j.als.20150502.03
Preliminary Phytochemical Screening of 27 Plants Species Use in Ethnoveterinary in Khartoum State, Sudan
Hatil H. EL-Kamali, Ahmed A. Elshikh
Dept. of Botany, Omdurman Islamic University, Omdurman, Sudan
Correspondence to: Hatil H. EL-Kamali, Dept. of Botany, Omdurman Islamic University, Omdurman, Sudan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In the present study, preliminary phytochemical screening of 27 plants used in Ethnoveterinary medicine was done for the qualitative analysis of various phytochemical studies such as carbohydrate, reducing sugars, monosaccharide, tannins, saponins, flavonoids, terpenoids, alkaloid, proteins, amino acid and anthraquinones. In both ethanolic and aqueous extracts were performed for each plant species under study.
Keywords: Phytochemical screening, Ethnoveterinary medicine, Qualitative analysis, Ethanolic, Aqueous extracts
Cite this paper: Hatil H. EL-Kamali, Ahmed A. Elshikh, Preliminary Phytochemical Screening of 27 Plants Species Use in Ethnoveterinary in Khartoum State, Sudan, Advances in Life Sciences, Vol. 5 No. 2, 2015, pp. 48-52. doi: 10.5923/j.als.20150502.03.
Article Outline
1. Introduction
- Phytochemical studies have attracted the attention of plant scientists due to the development of new and sophisticated techniques. These techniques played a significant role in giving the solution to systematic problems on the one hand and in the search for additional resources of raw materials for pharmaceutical industry on the other hand. Plant synthesizes a wide variety of chemical compounds, which can be sorted by their chemical class, bio synthetic origin and functional groups into primary & secondary metabolites. Knowledge of the chemical constituents of plants is desirable, not only for the discovery of therapeutic agents, but also because such informat ion be of value in disclosing new resources of such chemical substances [1]. Plants have been a common source of medicaments, either in the form of pure active principle or as traditional preparations and it are reasonable to use locally available plants knowledge on the plant phytochemistry provides a fundamental use of plants as a reservoir of chemical agents in the field of medicine [2]. The quantity and quality of phytochemicals present in plant parts may differ from one part to another. In fact, there is lack of information on the distribution of the biological activity in different plant parts essentially related to the difference in distribution of active compounds (or active principles) which are more frequent in some plant parts than in others [3]. Ethnoveterinary medicine (EVM) deals with people’s knowledge, skills, methods, practices and beliefs about the care of their animals. Ethnoveterinary knowledge is acquired through practical experience and has traditionally been passed down orally from generation to generation [4]. Ethnoveterinary practice to animal health care is as old as the domestication of various animal species [5]. In this study, various solvent extracts of 27 plant used in Ehnoveterinary medicine in Khartoum State were qualitatively screened for phytochemicals using standard tests.
2. Materials and Methods
2.1. Plant Material
- The samples of the candidate plants were purchased from Omdurman market and some were collected from Omdurman Islamic University field. plants used were identified and authenticated at the Herbarium, Botany Department. Faculty of Science and Technology, Omdurman Islamic University, by Hatil Hashim AL-kamali.
2.2. Plant Extraction
- The candidate plants were air-dried at room temperature (26°C), after which it was grinded to a uniform powder. The Ethanol and Aqueous extracts were prepared by soaking 10g each of the dry powdered plant materials in 50 ml of ethanol and water at room temperature for 48 hr. The extracts then were filtered, first through a Whatmann filter paper No. 42 (125mm) and then through cotton wool.
2.3. Phytochemical Screening of Extracts
- The ethanolic and aqueous extracts were used to perform the phytochemical screening by using standard method [6], for the detection of the following:
2.3.1. Carbohydrates (Molisch's Test)
- To the extract, 1ml of the Molishs reagent was added then along the walls of the test tube carefully conc H2SO4 was added. Formation of a brown ring at the junction of two liquids was observed.
2.3.2. Reducing Sugars (Fehling's Test)
- The extract was taken in a test tube, and the 1ml of the Fehling's solution (A and B) was added and boiled on the water bath. The solution was observed for the colour change reaction.
2.3.3. Monosaccharide's (Barfoed's Test)
- To the extract in a test tube, 1ml of Barfoed's reagent was added and boiled on the water bath. The solution was observed for the colour change reaction.
2.3.4. Tannins (Ferric Chloride Reagent)
- 0.5ml of the extract was boiled with 10ml of distilled water in a test tube and then, few drops of 0.1% Ferric chloride solution was added and the reaction mixture was observed for blue or greenish black colour change.
2.3.5. Saponins (Frothing Test)
- 0.5ml of the extract was added to 5ml of distilled water in test tube. The solution was shaken vigorously and observed for the stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken vigorously after which it was observed for the formation of an emulsion.
2.3.6. Flavonoids
- To the 0.5ml of the extract, was 5ml of distilled water added and then a piece of magnesium ribbon and 2ml of concentrated HCl was added. The reaction mixture was observed for the pink or red colour solution.
2.3.7. Terpenoids / Steroids (Salkowski Test)
- To 0.5ml of each of the extract, was 2ml of chloroform added and then 3ml of the concentrated H2SO4 was carefully added to from a layer. A reddish brown colouration of the interface indicates the presence of terpenoids / steroids.
2.3.8. Alkaloids (Wagner's Test)
- To 0.5ml of the extract, 2ml of Wagner's reagent was added and the reaction mixture was observed for the formation of reddish brown precipitate.
2.3.9. Proteins (Biuret Test)
- To 0.5ml of the extract, 2ml of Biuret reagent was added and the reaction mixture observed for the formation of violet colour solution.
2.3.10. Amino Acids (Ninhydrin Test)
- To 0.5ml of the extract, 2ml of the Ninhydrin was added and heated for few minutes and the reaction mixture was observed for the deep blue to pale yellow colouration.
2.3.11. Anthraquinones (Borntragor's Test)
- To 0.5ml of the extract, 5ml of dilute HCl was added and boiled on water bath for 10 minutes and filtered. Then the filtrate was extracted with carbon tetra chloride and the equal amount of ammonia was added. After shaking the reaction mixture was observed for the formation of pink– red colour in the ammonia layer.
3. Results and Discussion
3.1. Phytochemical Screening of Plants Extracts
- Phytochemical analysis of studied plants was performed for constituents: carbohydrates, reducing sugars, monosaccharides, tannins, saponins, flavonoids, terpenoids, alkaloid, proteins, amino acids and anthraquinones present in an investigated plant species (Table 1). All the candidate plants were found consisted of carbohydrate in both the ethanolic and aqueous extracts except the Citrullus colocynthis (seeds tar), but Maerva crassifolia (stem bark) extract hasn't given a positive carbohydrate test in the aqueous extract. Some plants were found free of reducing sugar in both ethanolic and aqueous extracts, such as Trigonella foenum-graecum (seeds), Cymbopogon schoenanthus (arial parts), Cucurbita pepo (seeds), Conocarpus erectus (leaves), Nicotiana rustica (arial parts) and Citrullus colocynthis (seeds). The number of candidate plants which were found consisting monosaccharide was ten out of twenty seven in the ethanolic extract and eleven in the aqueous extract. Only four plants contained tannins in both the ethanolic and aqueous extracts, namely: Camellia sinensis (leaves), Acacia nilotica (fruit), Acacia mellifera (stem park) and Conocarpus erectus (leaves). But for the Ziziphus spina-christi (leaves) the tannin were found in the ethanolic extract only. Eleven plant were found contained saponins in the ethanolic and eighteen in the aqueous out of twenty seven total. The existence of the flavonoids appeared in few of the candidate plants, Camellia sinensis (leaves), Hibiscus sabdariffa L (fruit), in both ethanolic and aqueous extracts, but for Acacia nilotica (fruit), Capsicum Frutescens (fruit) and Citrullus colocynthis (seed tar). The most Terpenoids abundant phytochemical in the twenty seven plants and its existence was clear in both ethanolic and aqueous extracts of twenty one out of twenty seven, for the remaining it appeared only in the ethanolic extract. The alkaloids were abundant in most candidate plant except only five, namely; Maerva crassifolia (stem park), Pennisetum orientale (seeds), Balanites aegyptiaca (fruit), Cucurbita moschata (seeds) and Conocarpus erectus (leaves). Protein appeared in 8 plants for both ethanolic and aqueous extracts, 3 plants for ethanolic and 3 for aqueous. Amino acids in all plants appeared in all candidate plants except for Coffea arabica (seeds), Cymbopogon schoenanthus (Arial part), Hibiscus sabdariffa L (Fruit), Acacia mellifera (stem park), Balanites aegyptiaca (Fruits), Ziziphus spina-christi (Leaves) and Conocarpus erectus (leaves). Anthraquinones appeared in seven plants for both extracts, four in ethanolic and three in aqueous extract. Phytochemical tests revealed that tannins, saponins, flavonoids, terpenoid, steroids and carbohydrates present; these secondary metabolites have been shown to have therapeutic activities in plants and function in a synergistic or antagonistic fashion for the treatment of diseases [7]. Tannins are reported to possess physiological astringent and haemostatic properties, which hasten wound healing and ameliorate inflamed mucus membrane and also inhibit the growth of microorganisms by precipitating microbial proteins and making nutritional proteins unavailable for them; they form irreversible complexes with proline rich proteins, resulting in the inhibition of the cell protein synthesis. They have important roles such as stable and potent anti-oxidants [8-11]. They act as binders and for treatment of diarrhea and dysentery [12]. Saponins have expectorant action which is very useful in the management of upper respiratory tract inflammation; saponins present in plants are cardiotonic in nature and are reported to have anti-diabetic and anti-fungal properties [13-15]. Flavonoids and Phenolics having animportant role in control & prevention of tissue damage by activated oxygen species [16], they are currently of growing interest owning to their supposed properties in promoting health (anti-oxidants) [17].
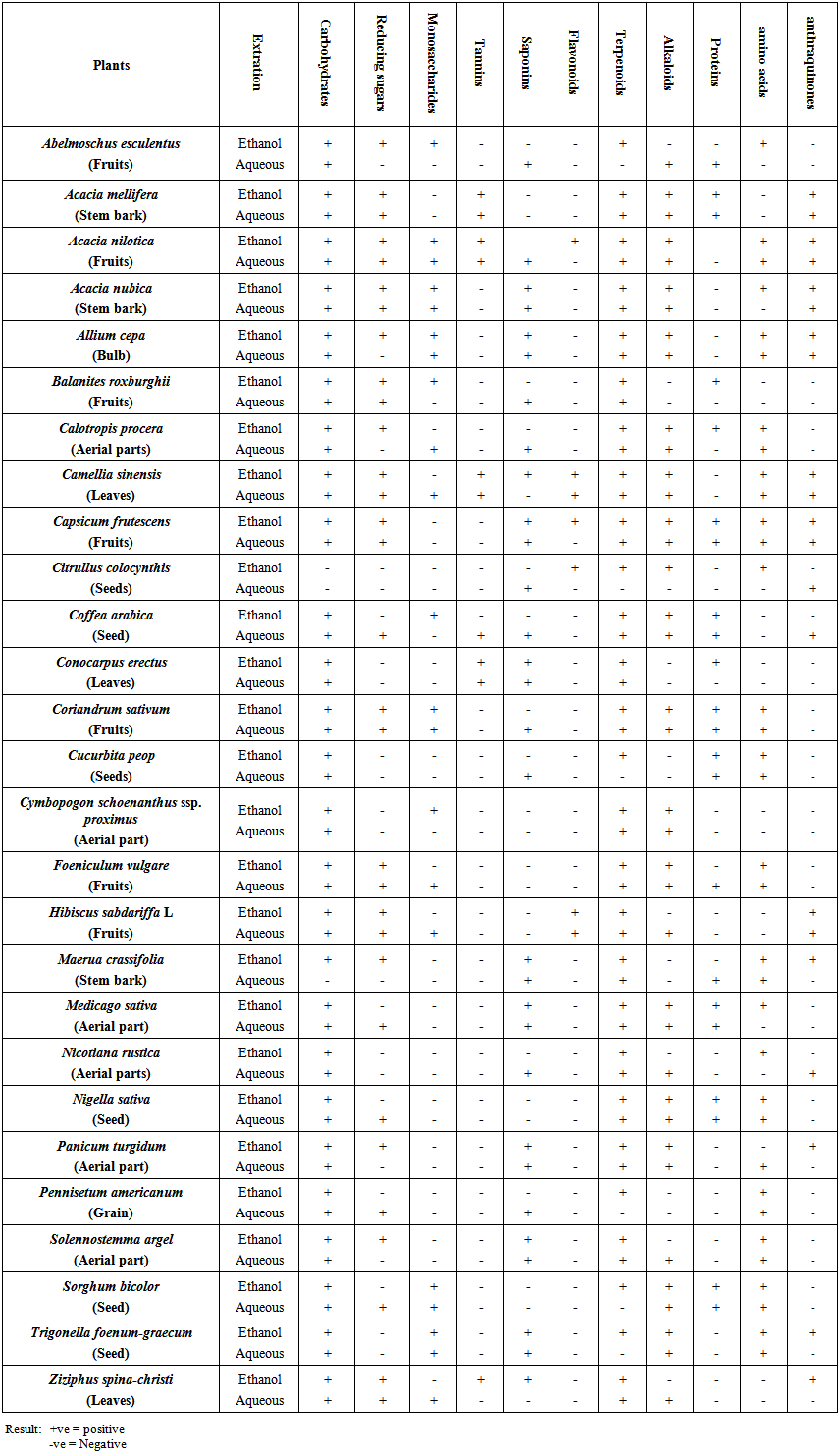 | Table 1. Phytochemical screening of plants used in traditional medicine veterinarian |
4. Conclusions
- This study of the preliminary phytochemical analysis revealed that these phytocompounds are mainly present in the ethanolic and aqueous extracts as shown in Table (1). So the ethanolic extract of the samples of plant material were found to contain the required major phytocompounds and other nutritive compounds needed by the pharamaceutical companies as well as in food supplements. The quantitative analysis of these phytocompounds will be an interesting area for further study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML