-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(2): 39-47
doi:10.5923/j.als.20150502.02
Changes in the Lipid Profile of Clupea harengus Fillet & SHB (Skin, Head and Bones) after Different Heat Treatment
O. T. Adeyemi1, O. Osilesi1, O. O. Adebawo1, F. D. Onajobi1, S. O. Oyedemi2
1Department of Biochemistry, Bencarson School of Medicine, Babcock University, Ilisan Remo, Nigeria
2Botany Department, University of Fort Hare, Alice, South Africa
Correspondence to: O. T. Adeyemi, Department of Biochemistry, Bencarson School of Medicine, Babcock University, Ilisan Remo, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Clupea harengus is a table fish locally called sawa in south west Nigeria. Present study was performed to assess the alteration in the lipid quality of processed Clupea harengus fillet and SHB (skin, head and bone) subjected to smoking (wood and coal) and poaching methods. Fatty acid analysis was done via standard analytical technique. The result showed that stearic and oleic acids had the highest concentrations among saturated and mono unsaturated fatty acids in both the fillet & SHB for all the processing methods. It was also revealed that samples of Clupea harengus recorded (p<0.05) increase in total saturated fatty acid (TSFA) in the fillet, but decrease (p<0.05) in the SHB with various heat treatments; whereas the same heat treatments reduced the components of total mono unsaturated fatty acids (TMUFA) and total essential fatty acid (TEFA) in both the fillet & SHB samples. It was found that levels of ω3 / ω 6 fatty acid ratio (often used as biomedical index) was desirable i.e. ratio 2:1 in all processed fillet, but fluctuated in the SHB. Overall best processing method was the wood smoked for the fillet sample, followed by the charcoal smoked for the SHB. Nonetheless since the SHB has shown great promise as a possible source for essential fatty acids, it could be used to fortify other feeds / food stuff, though considered waste left-over.
Keywords: Lipid profile, Clupea harengus, Processing methods, Fillet and SHB
Cite this paper: O. T. Adeyemi, O. Osilesi, O. O. Adebawo, F. D. Onajobi, S. O. Oyedemi, Changes in the Lipid Profile of Clupea harengus Fillet & SHB (Skin, Head and Bones) after Different Heat Treatment, Advances in Life Sciences, Vol. 5 No. 2, 2015, pp. 39-47. doi: 10.5923/j.als.20150502.02.
Article Outline
1. Introduction
- Fish and other marine species give rise to the economy of most countries; and is in increasing demand in Nigeria due to high population growth rate, as well as the increasing nutritional income cost of meat and other sources of animal protein [1-2]. The relatively high percent consumption of fish has been attributed to greater availability of this product at relatively cheaper prices [3]. It has been considered to contain high nutritive value due to the presence of essential mineral, amino acid and fatty acid compositions [4-5] Fish meat contains significantly low lipids, cholesterol content, but higher amount of water than beef or chicken [6], and is often recommended for consumption especially among the adult population [7]. Findings of clinical and epidemiological research suggest that eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, found only in fish and seafood, have extremely beneficial properties for prevention of human coronary artery disease and may reduce the risk of mortality from cardio- vascular disease in people who have already experienced a cardiac event [8-9]. In addition, fish oil helps to prevent brain aging and Alz- heimer’s disease [10]. Many health experts suggest that two to three servings per week of seafood should be consumed in order to meet the recommended level of essential fatty acids for pregnant women, children and elderly people [11].Nonetheless, eating large amounts of seafood over a long span of time has been reported to increase the risk of mercury poisoning. Children and fetuses are especially vulnerable. For this reason, women who are pregnant or nursing, women who may become pregnant, and young children are advised to completely avoid eating swordfish, shark, king mackerel, and tilefish; also to eat no more than 6 ounces per week of white (albacore) tuna; and eat no more than 12 ounces per week of other fish and shellfish [12]. This is because sea fish like tuna, mackerel, swordfish or striped bass have been reported to have high methylmercury contents. Some natural mecury sources are from volcanic eruptions, while some species of bacteria produce methylmercury, as a by-product of their respiration. This has been observed in bacteria living in seafloor sediments along coasts and on continental shelves [12]. Fish is rarely eaten raw thus it is usually cooked in different ways before consumption. During cooking, chemical and physical reactions take place that improve or impair the food nutritional value [13-16]. Moreover, this effect is also dependent on the type of cooking [17-18]. The effect of different processing and cooking methods on nutritional composition of different species of fish have been studied [19-22]. Different processing and drying methods have varing effects on nutritional composition of fish [23]. This is because heating, freezing and exposure to high concentration of salt leads to chemical and physical changes and increased digestibility, due to protein denaturaton, but the content of thermolabile compounds and polyunsaturated fatty acids in often reduced. Therefore, the quality of fish dried using different methods cannot be the same.There have been many notable studies dealing with the tissue changes of various fish species during postmotem handling and storage [24-34]. However to the knowledge of the researcher, no research related to the changes in fatty acid profile of Clupea harengus Fillet & SHB (skin, head & bone) after different processing methods has been encountered yet. Therefore the objective of this work was to carry out a comparative study on the effect of poaching, wood (Acacia seyal and Citrus lemon) and charcoal smoking on the fatty acid profile of Clupea harengus. Also noting that the raw and processed SHB (skin, head and bones) of this fish, often considered insignificant by majority of the consumers & manufacturers, may contain vital nutritive values for animal feed.
2. Procedure
2.1. Sample Collection
- The mean length and weight of Clupea harengus were; 30.52 ± 0.22 cm and 197.66 ± 3.67g respectively. Freshly purchased fish, packed in ice polystyrene boxes were transported to the laboratory within 30 min. The fish was thoroughly washed and drained, placed on wire gauze and cooked by poaching or smoking (firewood or charcoal). Poaching of the fish was done according to the method described by USDA [35], modified by Larsen [36]. The procedure was followed without addition of any ingredient. T. trachurus weighing 7 kg was hot smoked using either firewood or charcoal in Altona smoke kiln as described by FAO/UN [37]. The smoking time, temperature and ambient conditions were monitored during the smoking operation. Smoking was terminated when fish was properly dried to an average moisture content of 10.41±0.02%, after 8 hours. The fish was turned at intervals and the smoked or poached fish samples kept in cane woven baskets, under laboratory conditions with no preservative, left to cool and subsequently packaged in low density and high-density polyethylene bag, sealed and then stored at 8°C for further use. The following acronyms were used in labelling each of the processed fish samples; CSSF: charcoal smoked sawa fillet; WSSF: wood smoked sawa fillet; PSF: poached sawa fillet; RSF: Raw sawa fillet.
2.2. Analytical Method
- After poaching and smoking, fish samples were dried in a microwave oven to constant weight at 60℃, and the flesh of each fish was separated from its bones, skin and head. The skin, head and bones were collectively homogenized while the fillet alone was homogenized using a kitchen blender and analyzed to determine the proximate composition in each of the fish samples on dry matter basis.
2.3. Fatty Acid Determination
2.3.1. Oil Extraction
- Crude fat was extracted from the finely ground fish samples using the Soxhlet extraction method described by the AOAC, [38]. 2.0 g of moisture free sample was weighed into a fat free thimble, plugged with cotton wool and then introduced into the extraction tube. A clean dry boiling flask was weighed (W1) and 250 ml of 40- 60°C petroleum ether was introduced into the flask and sample was extracted for 6 h continuously adopted according to the EPA 3540 Method [39]. The extract was concentrated in a rotary evaporator (RE-100, Stone Staffordshire, and England) at 60°C to 2 ml. This was repeated for other samples. Then the remaining solvent was removed from the extracted oil by placing the flask in the fume hood at 25°C for 45 min. and weighed (W2). The percent crude fat was calculated by the following formula:
 Where W1 = weight of empty flask; W2 = weight of flask and fat deposit.
Where W1 = weight of empty flask; W2 = weight of flask and fat deposit. 2.3.2. Fatty Acid Methyl Ester (FAME) Analysis
- Methyl esters were prepared by trans methylation using 2M KOH in methanol and n-heptane ding to the method described by Ichihara et al. [40] modified by Suseno et al. [41] 2011). The extracted fish oil (0.1 g) was dissolved in 2 ml hexane. After the addition of 4 ml of 2M methanolic KOH, the tube was votexed for 2 min at room temperature, and then centrifuged at 4000 rpm for 10 min. The hexane layer was then taken for analysis by gas chromatography (GC). The fatty acid composition was analysed by GC Shimadzu 2010 with an auto sampler (Shimadzu, Japan) equipped with a flame ionization detector and a fused silica capillary SGE column (30 m x 0.32 mm, ID x 0.25 lm, BP20 0.25 UM, USA). The oven temperature was 140°C, held for 5 min, raised to 200°C at a rate of 4°C/min and to 220°C at a rate of 1°C/min, while the injector and the detector temperatures were set at 220°C and 280°C, respectively. The sample size was 4μl and the carrier gas was controlled at 16 psi. The split used was 1:100. Fatty acids were identified by comparing the retention times of FAME with a standard 37 component FAME mixture (Supelco). Three replicate GC analyses were performed per sample and the results were expressed in GC area % as a mean value and ± standard deviation.
2.4. Statistical Analysis
- The data from all the analyses were collected and statistically analyzed and expressed as the mean ± standard error (s.e.) (n=3), the significant differences between means were compared for each group of rats using the least significant difference test after ANOVA for one-way classified data. SPSS 14.0 [42] statistical tool was used to analyze data obtained. Results were considered statistically significant at a level of p < 0.05, chosen as the minimum for significance with Duncan’s multiple range test [43].
3. Results
- The fatty acid (F.A) composition of raw and processed fillet and SHB are shown in Tables 1 & 2 as well as Figures 1 & 2. A total of 24 F.A (fillet) and 17 F.A (SHB) were identified. Tables 1 & Figure 1 gives a summary of levels of the total saturated fatty acid (SFA), monounsaturated fatty acid (MUFA) & polyunsaturated fatty acid (PUFA) in the fillet & SHB. Figure 1 indicated that SFA and MUFA were the predominant fatty acids in both the fillet & SHB. Processing methods decreased (p < 0.05) in the levels of total saturated (SFA) mono and polyunsaturated fatty acid (PUFA) in both the fillet and SHB. The principal fatty acid among the unsaturated fatty acids was stearic, which increased with heat application i.e. 39.60% (WSSF) = 39.40% (CSSF) = 39.20% (PSF) compared to 38.80% (RSF). The oleic acid (C18:1) had the highest concentration among the mono unsaturated fatty acids, which also increased with heat application i.e. 39.09% (WSSF)> 35.41% (CSSF) = 35.93% (PSF) compared to 38.80% (RSF). While α–linolenic acid (C18:3) was highest amongst the essential fatty acid i.e. 39.20% (WSSF) > 32.40% (PSF) > 22.00% (CSSF) compared to 38.93% (RSF). The summation of ω 3 / ω 6 fatty acids ratio (Σ ω 3/ ω 6) were 1.95, 1.46 & 0.99 for WSSF, PSF and CSSF compared to 1.87 in the RSF respectively. The overall best method in the fillet was the wood smoked process.
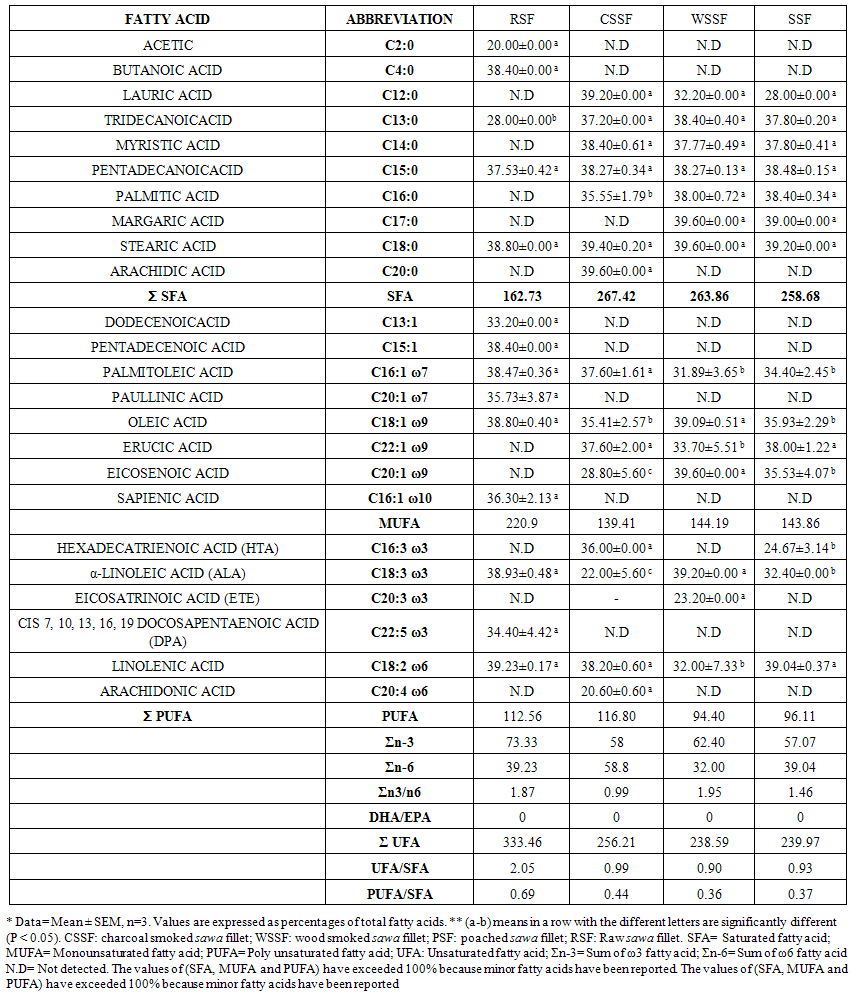 | Table 1. Fatty Acid Composition (%) of Raw and Processed Fillet* |
 | Table 2. Fatty Acid Composition (%) of Raw and Processed SHB* |
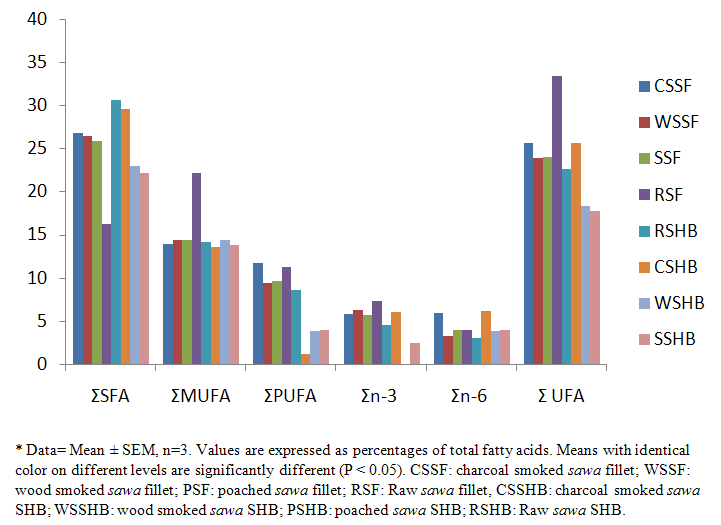 | Figure 1. Summation of Fatty Acid Composition for Both Raw and Processed SHB* |
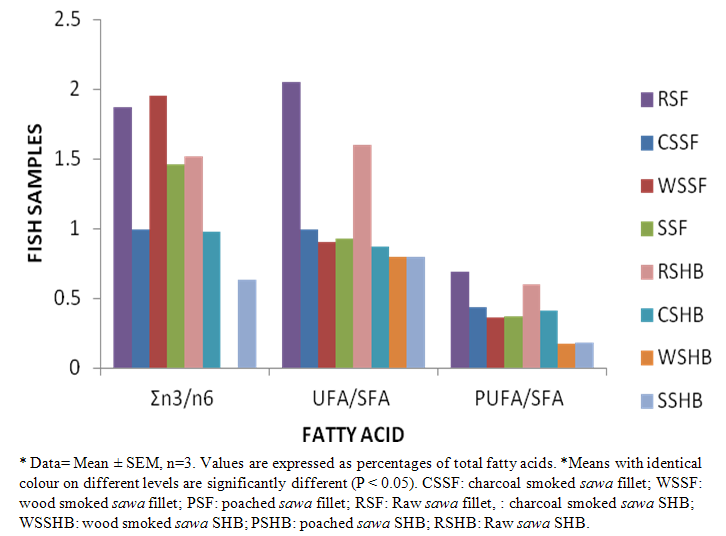 | Figure 2. Summation of Fatty Acid Composition for Both Raw and Processed SHB* |
4. Discussion
- The fatty acid compositions after application of different heat treatments are presented in Table 1. The most predominant fatty acid in C. harengus after the application of different heat treatment was stearic acid (C18:0) which ranged from 39.60% in the wood smoked fillet to 38.80 in the raw filet and 39.40% in the raw SHB to 35.52% in the charcoal smoked SHB. These findings disagrees with the results obtained for Oreochomis mossambicus & Oreochomis niloticus fish as reported by Dhanapal et al.[44] & Agbo et al, [5]. The highest concentration recoreded in oleic acid (C18:1) among the unsaturated fatty acids concors with the report of Unusan [45], who has also observed that oleic acid was the most concentrated unsaturated fatty acid in rainbow trout (Oncrohynchus mykiss) species after cooking. While the variations observed in α–Linolenic acid, an essential fatty acid in both the fillet & SHB concors with the report of Agbo et al, [5] for Oreochomis niloticus. It has been reported that high temperature processing can potentially damage polyunsaturated fatty acid (PUFA) [46-47]. Shahidi and Miraliakkbari [48] also reported that n–3 polyunsaturated fatty acids are very susceptible to oxidation which not only affects the sensory attributes of the foods but also contribute to many diseases in human being. The slight (p<0.05) changes in fatty acids profile in total mono unsaturated fatty acids (TMUFA) and total essential fatty acid (TEFA) in both the fillet & SHB samples samples with different smoking methods may be attributed to effect of thermal cracking on the fatty acids reported by Domiszewski et al. [49] and Finot [13].The average value of TSFA in this report was (p<0.05) higher in both the fillet & SHB than the 53.94% for tilapia fish (Oreochomis mossambicus) reported by Dhanapal et al. [43]. TUFA consists of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). TMUFA was observed to be higher (p<0.05) than the TPUFA in the fillet. TMUFA ranged from 139.41% in the coal smoked fillet to 144.19% in the wood smoked fillet. While, TMUFA in the SHB was (p<0.05) higher than in the fillet i.e. TFUMA ranged from 221.45% in the poached SHB to 295.67% in the coal smoked SHB. The TMUFA obtained in this study is significantly higher than the value obtained in rainbow trout (Oreochomis niloticus) & tilapia (Oreochromis niloticus) after cooking as reported by Unusan [45] & Agbo et al [5]. TPUFA constitutes linoleic (C18:2), linolenic (C18:3) and arachidonic (C20:4) acids. Findings in current study showed that the total polyunsaturated fatty acids (TPUFA) ranged from 94.40% in wood smoked fillet to 116.80% in the coal smoked fillet, but TPUFA was 38.53% in the poached SHB to 120.70% in the coal smoked SHB. The amount of omega–3 PUFA (ω–3 PUFA) detected in raw and processed fillet of present study were; hexadecatrienoic acid (C16:3), α-linoleic acid (ALA) (C18:3), eicosatrinoic acid (ETE) (C20:3) and docosapentaenoic acid (DPA) (C22:5), while the omega–6 PUFA (ω –6) were linoleic (C18:2) and arachidonic acid (C20:4). ω–3 PUFA ranged from 5.0% in sample smoked with sawdust to 5.77% in sample. While ω-3 PUFA observed in both the raw & processed SHB were hexadecatrienoic acid (C16:3), α-linoleic acid (ALA) (C18:3) and docosapentaenoic acid (DPA) (C22:5); While ω–6 PUFA were linoleic (C18:2) and arachidonic acid (C20:4).The essential fatty acids like omega-6 and omega-3 are indispensable for the normal development of the body [5]. Eicosapentaenoic (EPA) is needed to make hormone like substances called prostaglandins, which are anti-inflammatory in nature. It is found in fish liver oil supplements and cold-water fish, such as salmon, trout, and tuna. Docosahexaenoic (DHA) is necessary for proper brain and nervous system development and visual function. It is found in cold-water fish and fish liver oil supplements and is also available in vegetarian supplements made from micro-algae. DHA has beneficial effects on cancer, cardiovascular diseases, inflammation, developmental disorders and psychiatric disorders [29]. All earlier observed FAs in addition to linoleic and α–linolenic acids in present study, were observed to be in significantly (p<0.05) high amount present in all the samples (fillet & SHB) of C. harengus, therefore the samples will participate well in these functions. The ratio of n–6 PUFA to n–3 PUFA and linoleic to α–linolenic acids are used as a biomedical index. The oleic and linoleic (O/L) acids ratio has been associated with high stability and potentiality of the oil for deep frying fat [50-51]. Hence results of this study indicated that C. harengus contained appreciable (p < 0.05) amount of the essential and non essential fatty acids in both the fillet and SHB, with the fatty acid having similar patterns for both varieties, (i.e. higher concentrations of ω-3 than ω-6 in both fillet and SHB). Although the composition of fish and fish derived product is recommended as a means of preventing cardiovascular and other diseases [51-52].An imbalance in the ratio of omega-6 to omega-3 fatty acids can be detrimental to the health. A very high omegs-6 to omegs-3 ratio promotes the pathogenesis of many diseases like cardiovascular disease, cancer, inflammatory and auto-immune diseases whereas a low ratio exerts suppressive effect. A ratio of 2:3.5 of omega-6 to omega-3 suppresses inflammation in rheumatoid arthritis patients and a ratio of 5:1 has a beneficial effect in asthma patients [52]. A ratio of 10:1 has adverse consequences. Therefore, a lower ratio of 2:1 of omega-6 to omega-3 is more desirable in reducing the risks of many chronic diseases due to our faulty dietary choices [52]. This high value is an indication that C. harengus species used in this study obtained in Nigeria is more nutritive and agrees with earlier reports of Agbo et al [44] 2014) for tilapia; Adeyemi et al., [32] kote & Regan [27].
5. Conclusions
- The study has presented data on the concentrations of saturated and unsaturated fatty acids in sawa (Clupea harengus) fillet and SHB subjected to different heat treatments. The results showed that processed sawa contained high level of polyunsaturated fatty acids making it a healthy low–fat food. The wide variability of nutrient profile and content between the processing methods observed in the present study strengthens the importance of producing data derived from a selection of effect of cooking methods. It was also revealed that various heat treatments enhanced the component of essential fatty acids. Charcoal smoking was the best method in terms of increase in essential fatty acids in both the fillet and SHB. Hence Clupea harengus SHB could be a veritable source of valuable ingredients for human food and animal feeds, especially if milled into flour and used to fortify food / feed stuff, it may be a valuable alternative food.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML