-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2015; 5(1): 18-26
doi:10.5923/j.als.20150501.03
Key Roles of Calreticulin and Calnexin Proteins in Plant Perception under Stress Conditions: A Review
Garg G., Yadav S., Ruchi, Yadav G.
School of Biotechnology, Gautam Buddha University, Greater Noida, India
Correspondence to: Garg G., School of Biotechnology, Gautam Buddha University, Greater Noida, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The ER is one of the largest membrane organelle in eukaryotic cells. It plays a vital role in a variety of cellular processes including Ca2+ storage and release, lipid and protein synthesis, protein folding and post-translational modifications, organelle–organelle communication and signaling. The ER has a sophisticated Quality Control (QC) system to eliminate improperly folded proteins from the secrete pathway. For this ER contains many luminal and integral membrane proteins; several chaperones and folding enzymes, that are associated with correct folding and assembly of newly synthesized proteins. Calnexin (Cnx) and Calreticulin (Crt) are the two special type of proteins of ER chaperone system. Calnexin (Cnx) is the integral membrane protein type-I, which coordinates the processing of newly synthesized N-linked glycoproteins. Cnx interacts with many nascent membranes and soluble proteins of the secretory pathway. Cnx deficient organisms develop severe complications because of improper folding of proteins. Crt is a unique ER luminal Ca2+ binding chaperone implicated to play a role in many cellular functions, including lectin-like chaperoning, Ca2+ storage and signaling, regulation of gene expression, cell adhesion, wound healing, cancer and autoimmunity. In this research paper, we have focused for the role played by both Cnx and Crt under stress conditions in plant. Further, observations were obtained for these proteins and their involvement in providing stress tolerance against different abiotic and biotic stresses.
Keywords: Endoplasmic Reticulum, Protein Folding, Calreticulin, Calnexin, Ca2+ Homoeostasis
Cite this paper: Garg G., Yadav S., Ruchi, Yadav G., Key Roles of Calreticulin and Calnexin Proteins in Plant Perception under Stress Conditions: A Review, Advances in Life Sciences, Vol. 5 No. 1, 2015, pp. 18-26. doi: 10.5923/j.als.20150501.03.
Article Outline
1. Calnexin (Cnx) and Calreticulin (Crt): ER Chaperone System
- The ER is one of the largest membrane organelle in eukaryotic cells which contains the large concentration of chaperones required for an optimal environment for protein folding, modification and assembly. The ER plays a vital role in a variety of cellular processes including Ca2+ storage and release, lipid and protein synthesis, protein folding and post-translational modifications [1-3]. The ER contributes for organelle–organelle communication and signaling, including ER stress-dependent activation of transcriptional processes, Ca2+ signaling and communication to the Ca2+ channels of plasma membrane and ER associated degradation (ERAD).Researchers had defined ER as a multifunctional organelle which is able to detect and integrate incoming signals, modulate its own luminal dynamics and generate output signals in response to environmental changes [4]. The ER has a sophisticated Quality Control (QC) system to eliminate improperly folded proteins from the secrete pathway. QC is a surveillance mechanism that permits only properly folded proteins to exit the ER when they en-route to other intracellular organelles or to the cell surface [5] Fig. 1.
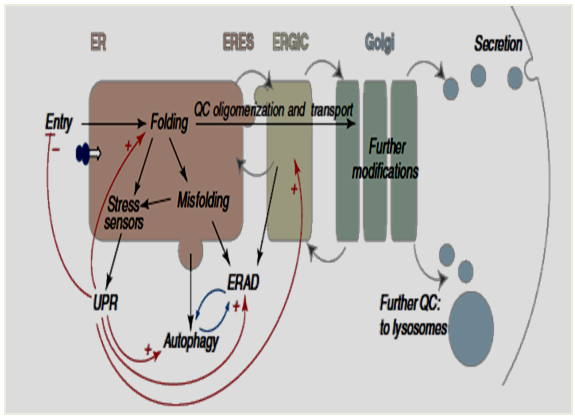 | Figure 1. Secrete Pathway showing downstream of the ER induced by unfolded protein response (UPR) |
1.1. Unfolded Protein Response of Calreticulin for Preventing ER Stress
- We observed that Crt is highly preserved ER protein in the plants. Till now research had been done which Initially, Crt has proposed in skeletal muscle cells [11]. Further, Crt has been characteristic for the cells found in the mammals. Functionally it had been associated with protein folding indirectly [12] and Ca2+ homeostasis [13-15] in plants. Crt has considered being the major site of calcium storage in the ER with its acidic C-domain binding 20–50 moles of Ca2+ per mole of protein [14]. C-domain transformants have increased total calcium by 9–35% compared to controls. The mRNA for Crt is most abundant in the tissues which are active in secretion, in vasculature during developing and germinating of the seeds in floral organs [16]. Crt operates in the unfolded protein response to prevent ER-stress, components of which are differentially regulated at mRNA level via PP2A-B’γ [(PP2A: protein phosphatase 2A activity) and (B’γ: regulatory subunit of PP2A)]. Controlled protein de-phosphorylation by protein phosphatase 2A regulates diverse signaling events in plants. Specific, B’γ regulatory subunit of PP2A mediates basal repression of immune reactions in Arabidopsis thaliana. Knock-down PP2A-B’γ mutants display constitutive defense reactions and premature yellowing conditionally under moderate light intensity. Trotta et al [17] has been reported that de-phosphorylation of Crt 1 is mediated by PP2A-B’γ and in PP2A-B’γ, strong phosphorylation of Crt 1 take place that may partially imbalance the quality control of protein folding, which eliciting ER-stress and premature yellowing in leaves.
1.2. Calnexin: ER Type-I Integral Membrane Protein
- Calnexin (Cnx) is the another major type-I integral membrane protein [19] in the endoplasmic reticulum, which coordinates the processing of newly synthesized N-linked glycoproteins with their productive folding [20-21]. The Cnx family of molecular chaperones is conserved among plants, fungi, and animals. In plants, Cnx is a new type of molecular chaperone that interacts with many nascent membranes and soluble proteins of the secrete pathway. Cnx deficient organisms develop severe complications because of improper folding of proteins and consequently ER stress [22]. Cnx binds Ca2+ and is a similar in sequence of Crt [23]. Phosphorylation of cytoplasmic tail of Cnx controls the sarco-endoplasmic reticulum calcium ATPase and thus, it controls the movement of Ca2+ in and out of its store-house, i.e. ER [22].
2. Targeting and Retention of Crt and Cnx in the ER Lumen through Structural and Functional Domains
- Crt represents a 46 kDa protein with an N-terminal cleavable amino acid signal sequences and a C-terminal tetrapeptide KDEL-ER retrieval signal, usually lys-asp-glu-leu [KDEL: Lys-Asp-Glu-Leu endoplasmic reticulum protein retention receptor 1, also known as KDELR1] [24, 25]. These are specific amino acid sequences, responsible for targeting and retention of Crt in the ER lumen. Depending on species, Crt may have one or more potential N-linked glycosylation sites. Cnx is a 90kDa type I integral protein [18] that appears variously as a 90kDa, 80kDa or 75kDa band on western blotting depending on the source of the antibody of the ER. Structural predictions of Crt and Cnx suggest that the proteins have at least three domains: N, P, C [24, 26, 27] (Fig. 2). The N-terminal part of the protein, encompassing the N- and P-domain, has the most conserved amino acid sequence. Careful examination of the exon–intron organization of the Crt gene suggests that the central P-domain of the protein may be encoded by exons 5, 6 and 7, whereas the first four exons and the last two exons may encode the N- and C-domain of the protein respectively.
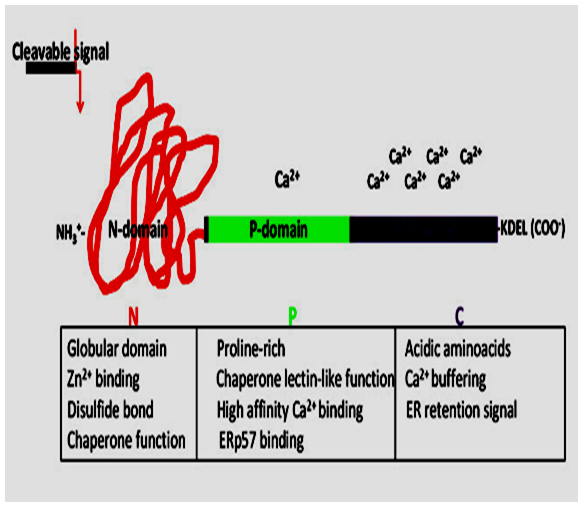 | Figure 2. Structure and Function of Calreticulin Domain |
2.1. N, P, and C Domains
- The N domain has been identified as calcium binding globular domain. It showing extremely conserved amino acid sequence [28]. The N-domain of Calreticulin forms a stable core that is resistant to proteolysis in the presence of Ca2+ [4]. In vitro studies indicate that the polypeptide and oligosaccharide binding regions are located in the N- and P-domain of Calreticulin [29]. Kapoor et al. [30] has identified two amino acid residues in Calreticulin’s globular N-domain (Tyr109 and Asp135), that abolish interaction of the protein with oligosaccharides. A number of additional residues might also be involved in sugar binding to the protein, including Lys111, Tyr128 and Asp317 [31-32]. In Cnx, six amino acids residues are reside in the globular N-domain. These amino acids are important for oligosaccharide binding (Tyr156, Lys167, Tyr186, Met189, Glu217 and Glu426) [33]. The middle portion of Crt and Cnx, named P-domain, rich in proline and providing potential flexibility to this region. The P-domains in these two proteins contain pairs of repeats (repeat A, IXDPXA/ DXKPEDWDX, and repeat B, GXWXPPXIXNPXYX). There are three sets of AB repeats in Crt [24, 26] and four in Cnx [18]. These repeats amino acid sequences form an important structural backbone of the P-domain and may be involved in the lectin-like function of the proteins [34]. Studies reveal that the structure of the P-domain of Crt contains an extended region stabilized by three anti parallel β-sheets [35] that interacts with ERp57 [36-38]. P-domain of Cnx also forms a similar extended arm structure [39]. The C-domain of Crt contains a large number of negatively charged residues that are responsible for the Ca2+ buffering function of the protein. It binds over 50% of ER luminal Ca2+ [40] with high capacity (25 mol of Ca2+ per mol of protein) and low affinity (Kd =2 mM). The C-domain of Cnx is also an interesting region. It extends from the trans-membrane α-helix, contains an ER-retention amino acid sequence, and stretches of negatively charged amino acid residues that bind Ca2+ with moderate affinity [18, 41]. This region of Cnx may play an important role in the regulation of protein–protein interactions via specific phosphorylation [42-44].
3. Isoforms of Crt and Cnx in Higher Plants
- Generally, Calreticulins (Crts) have expressed a chaperone that fold newly synthesized proteins and regulates Ca2+ homeostasis [45]. The Crt family consists of two members in animals (Crt1, 2 and 3), and three members in higher plants (Crt1a, Crt1b and Crt3; 4). Additionally, modulation of Crt expression has revealed through Crt functions which are necessary for plant regeneration and resistance to various environmental stresses, [46, 47]. But the exact role of Crts processes remains intangible. Interestingly, a recent report revealed that the third Crt isoform, Crt3, retains defective forms of the brassinosteroid receptor BRI1 in the ER in Arabidopsis [48]. Studies showed that neither the two other Crt isoforms, nor the membrane-spanning Crt. Homology Cnx, could retain the defective BRI1 suggesting functional specialization of the Crt3 isoform. Subsequently, several reports revealed that Crt3 also appears to mediate folding of the elf18 responsive EF-Tu receptor (EFR) associated with Pathogen-Associated Molecular Patterns (PAMPs) in Arabidopsis [49-50]. Additionally, Crt3 don’t harmonize Crt1a and Crt1b, which are double mutant phenotypes, showing corroborate diverged functions of the Arabidopsis Crt3 isoform [51]. Hence, it is clear that the Crt3 isoform perform different functions as compared to Crt1a and Crt1b in Arabidopsis. Indeed, while the Arabidopsis Crt1a and Crt1b genes appear co-expressed with many ER genes. Only one of the three forms of the Crt of the plant Arabidopsis thaliana interacts with a mutated form of BRI1 [48]. Additionally, we found perfect similarity between Arabidopsis Crt1 and Crt2 and have homologs in non plant organisms, but the BRI1-interacting Crt3 seems to be a plant-specific form, with orthologs in higher and lower plant species [52]. Initially, Cnx has characterized in Arabidopsis. Further, Cnx characterized in cloned and well characterized from pea. In Arabidopsis, three different Cnx has been reported (AtCnx1, AtCnx2 and AtCnx3) while rice encodes only one (OsCnx) [53], showing its functional divergence in different plant systems. Sarwat et al. [53] stated that dehydration tolerance given by Oryza sativa Cnx (OsCnx) appears to be ABA-dependent pathway. Oryza sativa Cnx (OsCnx) has revealed constitutive expression at various developmental stages and various tissues, thereby proving its requirement throughout the plant development. Further, researchers have observed that expression of OsCnx under various stress conditions gives an insight of the crosstalk accessible between ER stress and abiotic stress signaling.
4. The Calnexin/Calreticulin Cycle (Cnx/Crt)
- In the biosynthesis of glycoprotein bearing N-glycans, the oligosaccharide Glc3Man9GlcNAc2 is attached to the asparagines (N) residue contained in the consensus sequence N-x-S/T of newly synthesized polypeptide in the ER. Two glucoses are removed by glucosidases I and II after transfer of the core oligosaccharide (Glc3Man9GlcNAc2) to the nascent chain of the protein. This generates a monoglucosylated (Glc1Man9GlcNAc2) glycoprotein that can interact with Cnx and Crt. Both chaperones associate with the thiol-disulphide oxidoreductase ERp57 through an extended arm-like domain. During the catalysis of disulphide-bond formation, ERp57 forms inter-chain disulphide bonds (S–S) with bound glycoproteins. Cleavage of the remaining glucose by glucosidase II terminates the interaction with Cnx and Crt. On their release, correctly folded glycoproteins can exit the ER. However, if the glycoprotein are not properly folded, the terminal glucose is once again attached by the action of UDP-glucose: glycoprotein glucosyl transferase (UGGT), which discriminates between folded and unfolded substrates [54]. If the protein is permanently misfolded, the mannose residue in the middle branch of the oligosaccharide will be removed by ER 1, 2-mannosidase I. This leads to recognition by the ER degradation-enhancing 1, 2-mannosidase-like protein (EDEM), which probably targets glycoproteins for ER-associated degradation (ERAD) [6] (Fig. 3).
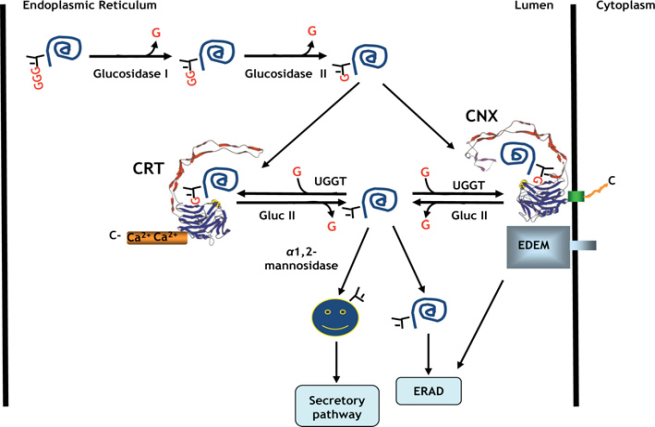 | Figure 3. Calreticulin/Calnexin Cycle |
5. Role of Calreticulin (Crt) and Calnexin (Cnx) under Different Stresses
- Throughout evolution, plants develop manifold defense mechanisms manifested through altered physiology to endure environmental stresses. Abiotic stress and combat challenges arising from biotic stress. Abiotic stresses include drought, excess water, salinity, heat, cold, wounding, exposure to Xenobiotics (now including chemical pollution) and UV radiation. Furthermore, plants are confronted by biotic stresses through microbial pathogens such as fungi, nematodes and bacteria. Traditionally, research has focused on single stress aspects. However, in their natural environment, plants have to adapt to numerous environmental stresses at the same time and different biotic stresses occurs at different stages of the plant’s life cycle. Plants perceive biotic and abiotic stresses via specific receptors. The stress recognition is usually followed by production of messengers such as reactive oxygen species (ROS) or Inositol-1,4,5-triphosphate (IP3), or modulated intracellular calcium (Ca2+). Production of these early messengers can be receptor mediated, however, in many abiotic stresses (e.g., drought, cold, salt stress) membrane over excitation can directly lead to ROS generation. The messengers also seem to influence each other via IP3 gated calcium channels. Receptors, ROS or Ca2+ dependent signals initiate specific phosphorylation cascades (e.g., mitogen activated protein kinases - MAPK, calcium dependent protein kinases - CDPK) and finally interact with the promoter regions of transcription factors (TF) and their responsive genes. The regulation of gene transcription can additionally depend on the activity of plants hormones and other regulatory molecules (e.g., abscidic acid - ABA, salicylic acid - SA, jasmonic acid - JA, ethylene - ET). The plant hormones can also influence the ROS and Ca2+ levels and initiate a second round of signaling.The adaptation to various stresses has led to the development of common stress transduction pathways and includes, among others, the increased synthesis of secondary metabolites, Ca2+ fluxes, an oxidative burst and an overlapping set of stress responsive genes [55, 56]. In the most stress full situations, signals are being recognized by receptors, followed by second messenger generation e.g. reactive oxygen species, or ROS [57]. Secondary messengers can modulate intracellular calcium levels, often initiating a protein phosphorylation cascade, which leads to the activation of proteins, which are directly involved for cellular protection (Fig. 4).
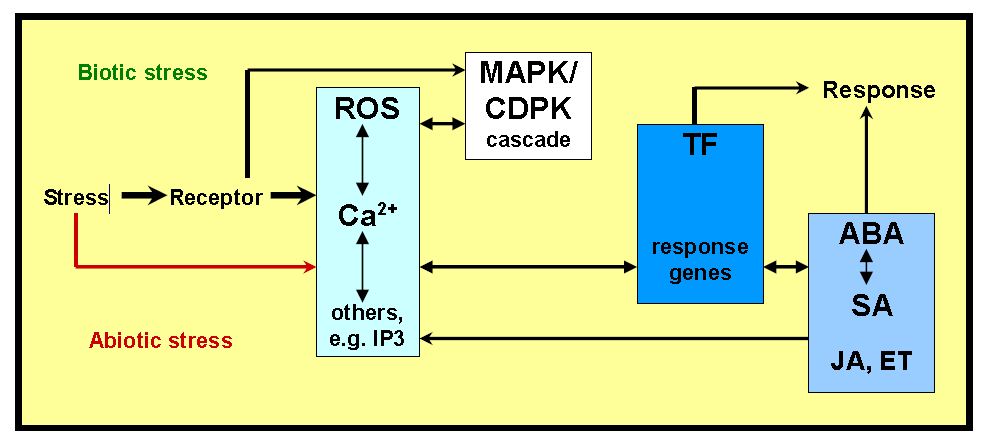 | Figure 4. Signaling Network in Plants under Biotic and Abiotic Stress |
5.1. Abiotic Stress
- Abiotic stress causes a diverse array of morphological, physiological, biochemical and molecular changes, leading to a decrease in plant growth and productivity [58]. Oxidative stress has occupied in various plant, results in the de-naturation of functional and structural proteins, leading to malfunctions in cellular responses [59, 60]. Secretory and trans-membrane proteins help in ER-folding. Misfolded parts of ER causes ER stress and trigger the unfolded protein response (UPR), which mediates refolding and the degradation of unfolded proteins. Many genes that exhibit up-regulated expression during ER stress have been identified, including the gene encoding Crt, an ER-localized Ca2+-binding protein [61, 62]. Crt is highly conserved in all multi-cellular eukaryotes including humans, nematodes, fruit flies and plants [63-67].Among plant Crts, the domain organization required for molecular structure and function is highly conserved and structurally similar to its mammalian counterparts [13-14, 16]. Based on sequence homology, these isoforms may form two distinct groups, Crt1/Crt2 and Crt3. Several Crt isoforms are present in plants, such as maize and barley [63, 68, 69]. There are three isoforms Crt1, Crt2 and Crt3 which has been identified in Arabidopsis. These proteins appear to modulate an array of cellular responses including Ca2+ dependent processes, the ER chaperone response and apoptosis [7, 12, 45, 70, 71]. Expression of plant Crts increases in response to a variety of environmental stimuli, such as gravi-stimulation [72], cold [47] and drought [46]. The Arabidopsis Crt isoform AtCrt1A (also known as AtCrt1) can substitute functionally for animal Crts, as a molecular chaperone and a modulator of Ca2+ homeostasis [73] in case of embryonic fibroblasts. Recently, it has been suggested that Arabidopsis Crt1, Crt2 and Crt3 mediate defense responses against viral and microbial pathogens [49, 50, 74, 75]. Although Crt1, Crt2 and Crt3 harbor significant levels of amino acid sequence identity, it appears that they present specific functions to cellular level [51, 76].Crt3 plays a specific role in brassinosteroid signaling, e. g. a loss of Crt3 function led to the accumulation of a substantial amount of the misfolded form of mutant BRI1 (leucine-rich repeat receptor-like kinase), despite the presence of Crt1, Crt2 and their membrane-localized homologues Cnxs [48, 77]. Both Sodium Cloride (NaCl) and high temperature stress markedly enhanced Crt mRNA levels in Brassica napus seedlings [78]. Crt and CDPK (calcium-dependent protein kinase) have associated with the development of tolerance to various stresses [79]. The activity of 47-kDa protein kinase is a cold-induced and dependent on Crt. Crt has differential expression and diverse function under abiotic stress conditions. Shaterian et al [80] studied the expression of Crt and concluded that expression of Crt appears to be involved in ABA-induced salt tolerance, which has to be regulated by the roots. Molecular cloning and characterization of wheat’s (Triticum aestivum L.) Calreticulin gene [46] indicated that it plays an important role in plant drought resistance, providing very useful information for the functional analysis of Crt and its implications in plant genetic improvement. Further, southern analysis suggests that the wheat genome contains three copies of TaCrt (full-length cDNA encoding calreticulin- like protein)/ or plants showed over-expresses TaCrt, exhibited enhanced drought resistance to water deficit [46]. Crts are also involved in the Arabidopsis response to water stress. As a regulatory protein it protects the plants from water stresses [81]. In case of osmotic or other abiotic stresses Cnx showing highly reducible accumulation in developing soybean roots [82]. Co-expression of a maize Calreticulin Crt1 (De-regulated expression of an Arabidopsis H+/ Ca2+ antiporter) mitigated these adverse effects via enhancing the Ca2+ content. Perhaps, co-expression of Crt and sCrt1 could alleviate the hypersensitivity to ion imbalance in tobacco plants. Furthermore, enhanced Crt expressions mitigated BER (blossom end rot) in sCrt1 expressing lines in tomato [83].
5.2. Biotic Stress
- Plants are permanently exposed to potential enemies including microbial pathogens (viruses, bacteria, and fungi), nematodes and insects, apart from environmental stresses. Naturally, the effect of pathogen challenge depends on the nature of the attack. Bio-tropic pathogens mainly withdraw nutrients from a living plant, while necro-tropic or hemibio-tropic pathogens induce cell death in host cells to access nutrients from the dying or decomposed host cells [84]. Most microbial pathogens enter the host cell, while others like mildew (e.g. Blumeria) or rust fungi (e.g. Hemelia) remain on the outside and grow on external surface for nutrient-absorption. During infection, when the integrity of the outer cell wall might be damaged by mechanical wounding then the pathogens from outside enters the cell. Calreticulins (Crts) play an important role in plant innate immune system, as they are required for the N immune receptor to provide complete defense against viruses (e.g. TMV) [85]. Recently, it has been suggested that Arabidopsis Crt1, Crt2 and Crt3 mediate defense responses against viral and microbial pathogens [49-50, 74-75]. Mi-CRT, (a calreticulin (Crt) secreted by the nematode into the apoplasm of infected tissues), plays an important role in infection. Mi- CRT suppressed plant basal defence during the interaction. Mi-CRT knockdown RNA interference and affects the ability of the Nematodes to infect plants. Furthermore, it is found that’s stably transformed Arabidopsis thaliana plants producing the secreted form of Mi-CRT, which were more susceptible to nematode infection than wild-type plants [86]. According to Matsukawa et al. [87] studies, Nicotiana benthamiana calreticulin 3a (NbCrt3a) is required for disease resistance of N. benthamiana against oomycetes pathogen Phytophthora infestans. NbCrt3a encodes an endoplasmic reticulum quality-control (ERQC) chaperone for the maturation of glycoproteins, including glycosylated cell-surface receptors [87].
6. Conclusions and Future Prospect
- Cnx and Crt are multifunction proteins which play major role of regulation and the relationship to the binding of other ligands need to be investigated. Their role in immunity has also been identified but their mode of action is still needs to be elucidate. Crt and Cnx plays monitoring role in stress signalling and tolerance management in plants. Knowledge about these proteins are of immense importance to crop scientists working towards development of stress resistance plants but further studies required for their species specific role. Overall, the ER chaperones have emerged as important molecules to understand stress signalling and tolerance management in plants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML