-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(4): 207-212
doi:10.5923/j.als.20140404.04
The Evaluation of Oxidative Stress and Depresion Levels in Patients with Inactive Behcet's Disease
Isil Gogcegoz Gul1, Birgul Elbozan Cumurcu2, Tongabay Cumurcu MD3, Gül Eryilmaz1
1Ass. Prof. Dr., Uskudar University, NP Istanbul Hospital, Istanbul/Turkey
2Assoc. Prof. Dr., Inonu University Faculty of Medicine, Department of Psychiatry Malatya/Turkey
3Assoc. Prof. Dr., Inonu University Faculty of Medicine, Department of Ophthalmology, Malatya/Turkey
Correspondence to: Isil Gogcegoz Gul, Ass. Prof. Dr., Uskudar University, NP Istanbul Hospital, Istanbul/Turkey.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Aim: We aimed to compare oxidative and antioxidative parameters in two groups consisting of Behçet’s disease (BD) patients and healthy controls and then in three groups consisting of BD patients with depression, BD patients without depression and healthy controls. Methods: A total of 34 BD patients with depression, 46 BD patients without depression and 53 control group subjects were included in our study. We measured the total antioxidant capacity (TAC), paraoxonase (PON), arylesterase (ARE), glutathione (GSH) antioxidants and malondialdehyde (MDA) oxidant levels in the blood samples obtained from the three groups.Results: When three groups consisting of the BD group with depression, BD group without depression and the control group were compared, a significant difference was found only for the TAC and GSH antioxidants and MDA oxidant. The two-way comparisons we performed to reveal the group that created the difference showed no significant difference in TAC, ARE, GSH antioxidants and MDA oxidant between the BD group with depression and BD group without depression, indicating that the difference between the three groups was due to the control group.Conclusions: The findings of this study show that the oxidant/antioxidant balance is disrupted in BD and oxidative stress is increased even in the inactive period. In addition, the deterioration in oxidative/antioxidative mechanisms in BD is not caused by the depression that often accompanies this disease. Evaluation of the effects of depression on the oxidant/antioxidant balance in BD is important in terms of providing guidance for future studies.
Keywords: Behçet’s disease, Depression, Antioxidant, Oxidative stress
Cite this paper: Isil Gogcegoz Gul, Birgul Elbozan Cumurcu, Tongabay Cumurcu MD, Gül Eryilmaz, The Evaluation of Oxidative Stress and Depresion Levels in Patients with Inactive Behcet's Disease, Advances in Life Sciences, Vol. 4 No. 4, 2014, pp. 207-212. doi: 10.5923/j.als.20140404.04.
Article Outline
1. Introduction
- Behçet’s disease (BD) was first identified by the Turkish dermatologist Hulusi Behçet as a triad of hypopyon, uveitis, and oral and genital ulcers [1]. Subsequent studies revealed that the disease was not limited to these three regions and showed a systemic course, involving many organs and systems such as the joints, and the gastrointestinal, urogenital, cardiac and central nervous systems. The basic pathology of this chronic systemic disease characterized by recurrent attacks and unknown cause is vasculitis [1, 2]. We defined inactive disease as the absence of oral aphthous ulceration, genital ulceration, or panuveitis, together with pseudofollicular or papulopustular skin lesions and negative pathergy test. The prestudy inactive period duration was at least 3 months for the patients included in the study. We included patients who did not use any treatment for Behçet’s disease or whose topical or systemic treatment (cyclosporine A, colchicine, steroids, etc.) had been discontinued at least 3 months previously in the study [1, 3].Evidence that free oxygen radicals play an important role in the pathophysiology of BD has been increasing in recent years. Free radicals are molecules that occur naturally as a result of normal metabolic pathways. The total oxidants and antioxidants of a healthy organism are in balance. If the endogenous and exogenous oxidants exceed a certain level or antioxidants are insufficient, the balance is disrupted in favor of the oxidants and oxidative stress occurs. It is suggested that free oxygen radicals may have a role in the pathogenesis of BD and can be used as a biological indicator of the disease [3-6].Psychosomatic symptoms are said to be present in 86% of BD patients at the time after diagnosis with depression developing afterwards [7, 8]. Psychiatric symptoms seen in BD are linked to organic disorders in the brain or to the corticosteroids used for treatment. Stress may play a role in the emergence and recurrence of BD with its negative effects on the immune system [8].Recent studies suggest that the disorder in free radical metabolism or deficiency in the antioxidant defense system have important roles in the pathology and clinical picture of depression [9, 10]. The oxidative/antioxidative parameters in BD and depression have been evaluated but the possible effects of depression during BD on oxidative/antioxidative parameters have not been investigated to the best of our knowledge. We therefore compared the oxidative / antioxidative parameters first in two groups consisting of BD patients and healthy controls and then in three groups consisting of BD patients with depression, BD patients without depression and healthy controls. Evaluation of depression on oxidant-antioxidant balance in BD is important in terms of providing guidance for future studies.
2. Material and Method
- A total of 80 BD patients in the inactive period (34 with and 46 without depression) diagnosed according to the diagnostic criteria of International Behcet’s Disease Group and followed up at İnönü University Eye Department's Behcet's Outpatients and 53 age-and sex-matched healthy control group subjects were included in the study. Patients with serious neurological, endocrine, vascular, urological and gynecological disease other than BD, and patients who had used any psychotropic medication within the last 3 months were excluded from the study. Subjects who had any systemic or psychiatric diseases or used any medication were excluded from the control group. Hormonal evaluations (LH, FSH, testosterone, estradiol, prolactin and thyroid hormones) were performed for the patients and those with any abnormal results were excluded from the study. The patients and the control group subjects were evaluated by a psychiatrist according to DSM-5. Patients with BD who are assessed according to DSM-5 by the same psychiatrist and diagnosed with any psychiatric diseases other than depression were excluded. As a result of psychiatric evaluation, Patients with BD were seperated into two groups as patients with depression and patients without depression. The demographic data of all participants were recorded and the Hamilton Depression Evaluation Scale (HAM-D), Hamilton Anxiety Evaluation Scale (HAM-A) were administered. Finally, total antioxidant capacity (TAC), paraoxonase (PON), arylesterase (ARE), glutathione (GSH) antioxidants and malondialdehyde (MDA) oxidant levels were measured in blood samples taken from all three groups.
3. Procedures
- A blood sample of 8-10 ml was drawn from the patient and control groups into biochemistry tubes with gel between 08.00 and 09.00 AM after at least 12 hours of fasting. Blood samples were centrifuged at 4000 rpm for 10 minutes and the serum removed. The removed serum was transferred to a dry sterile tube and kept at -80℃ until the day of study.
4. Biochemical Analysis
- Analysis of Serum PON, GSH, ARE Activity and TAC Levels Serum PON, GSH, ARE activity and TAC levels [11] were measured using an autoanalyser (Microgenics; MGC-240) with the Rel-Assay-Diagnostics brand commercial kits. Serum TAC levels were expressed as µmol TroloxEqv/L, GSH activity as µmol/L, and PON and ARE activity levels as U/L.Analysis of Serum MDA Levels MDA, the most important indicator of lipid peroxidation, was analyzed according to the method described by Ohkawa [12] The basic principle of the analysis is based on MDA in the environment entering into a reaction after being heated with thiobarbituric acid in an acid environment and creating a pink chromogen. The intensity of the pink color is directly proportional to the MDA concentration. Serum MDA levels were expresses as micromole/L.
5. Statistical Analysis
- SPSS (Statistical Package for Social Sciences) for Windows 16.0 software was used to analyze study data. Quantitative variables were expressed as mean ± standard deviation (SD) while qualitative variables were expressed as numbers and percentages. A normal distribution was found for quantitative variables by using the Shapiro Wilk normalcy test (p>0.05). Comparison of the patient and control groups was the unpaired T test, Pearson chi-square analysis and Fisher's Exact Chi-Square test. The three-way comparison of the BD patients with depression, BD patients without depression and healthy controls for oxidative and antioxidative parameters was with the one-way variance analysis (ANOVA) test and the two-way comparison with the Tukey method. The intragroup variables were tested with the Pearson Correlation Analysis. A p value <0.05 was accepted as statistically significant.
6. Results
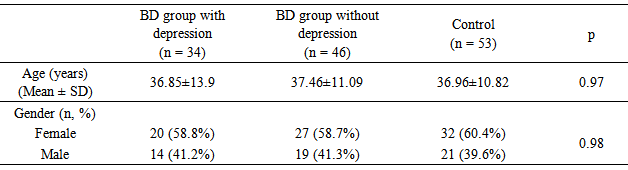
- A total of 80 BD patients consisting of 33 (41.2%) males, and 47 (58.8%) females were included in the study. Patients with BD consisting of 34 BD group with depression, and 46 BD group without depression. The mean age of the BD group was 36.83±10.84 years. The control group consisted of a total of 53 healthy individuals with 21 (39.6%) males and 32 (60.4%) females. The mean age of the control group was 36.96±10.82 years. No significant difference was observed between the patient and control groups in terms of age and gender (p>0.05). There mean age of the 34 patients with depression, 46 patients without depression and 53 control group was 36.85±13.9, 37.46±11.09 and 36.96±10.82 years respectively. No statistically significant difference was found between the BD groups with and without depression and control group in terms of age and gender (p>0.05) (Table 1).
|
|
|
|
|
7. Discussion
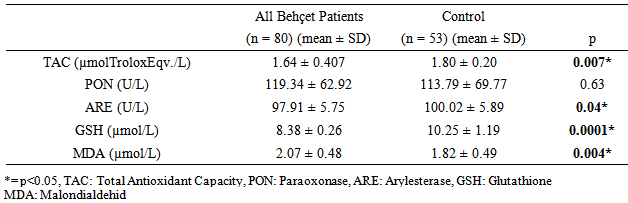
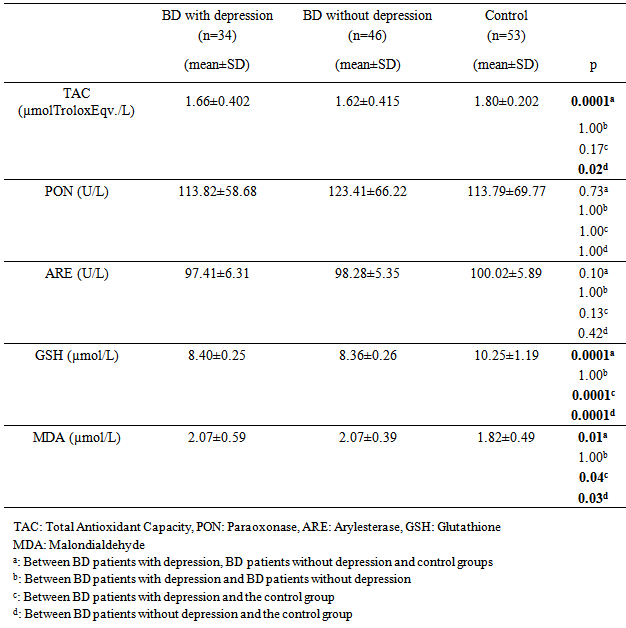
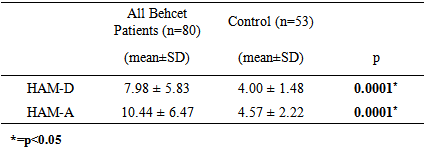
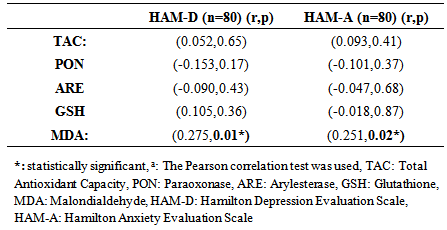
- Oxidative stress occurs as a result of an increase in the production rate or a decrease in the elimination rate of free oxygen radicals (FOR). The antioxidant defense system tries to eliminate the toxic effects of excessive FOR in the cells. Oxidative stress occurs when the antioxidant defense is insufficient and plays an important role in the development process of many disorders. Antioxidants have been reported to increase as a result of increased oxidative stress in BD [3-6]. Oxidant/antioxidant imbalance has been held responsible for the tissue damage in this disease [13, 14]. The findings of this study we conducted based on the hypothesis that the oxidative / antioxidative balance deterioration was more likely in Behcet patients with depression compared to Behcet patients without depression revealed significantly lower TAC, ARE and GSH antioxidant levels and significantly higher MDA oxidant levels in the BD groups than the control group. When three groups consisting of the BD group with depression, BD group without depression and the control group were compared, a significant difference was found only for the TAC and GSH antioxidants and MDA oxidant. The two-way comparisons we performed to reveal the group that created the difference showed no significant difference in TAC, ARE, GSH antioxidants and MDA oxidant between the BD group with depression and BD group without depression, indicating that the difference between the three groups was due to the control group. These findings suggest that the disorder in oxidative/antioxidative mechanisms in BD is not associated with depression.Recent studies on BD pathogenesis have focused on neutrophil functions. The tissue destruction including vascular and endothelial cell damage seen in BD is linked to the release of neutrophil lysosomal enzymes to the extracellular environment and to the excessive FOR production by active neutrophils [13, 15]. It is suggested that FOR production increases in stimulated neutrophils in BD in the active period and increases tissue destruction [13]. The antioxidant capacity has been reported to decrease due to the tissue destruction in BD caused by excessive FOR production, with the antioxidant capacity showing an inverse relationship with the severity of the disease [16]. A study reports that there is an increase in MDA oxidant and the oxidant-antioxidant balance is disturbed in BD. MDA levels in BD are said to cause the suppression of certain antioxidant enzymes in the same study [17]. The total antioxidant capacity in BD patients has been found to be significantly lower than in healthy individuals in many studies [5, 6, 18]. The levels of the TAC, ARE and GSH antioxidants were lower but the MDA levels were higher in BD patients than the control group in our study, supporting other studies reporting high MDA levels suppressing the antioxidant enzymes. BD patients in both the inactive and active periods have been found to have high MDA oxidant levels [5]. Türkmen et al. [19], Köse et al. [20], Sandıkçı et al. [21] conducted three studies and compared Behcet patients in the active and inactive periods in terms of oxidative stress. They found the MDA levels in patients in the active period to be increased compared with the inactive period. This increase in MDA level has been thought to support the presence of oxidative stress in BD and explain the low levels of GSH antioxidant activity in patients in the active period. The increase of the oxidant parameter MDA and decrease of antioxidant parameters despite BD patients being in the inactive period in our study may show that FOR increases in both periods in BD and oxidative tissue damage continues as the antioxidant defense system is suppressed.Another striking finding in our study was the lack of a significant difference in the oxidant and antioxidant parameters between the BD group with depression and BD group without depression. Cumurcu et al. [22] found total oxidative capacity and oxidative stress index to be higher than the control group in a study conducted in patients diagnosed with depression. These parameters were shown to decrease after treatment in this study. Sarandol et al.9 found TAC levels to be low in patients diagnosed with depression. Similar results have been reported in many studies conducted on the oxidative/antioxidative mechanisms in patients diagnosed with depression [9, 23, 24]. We have shown BD and depression to similarly affect oxidative / antioxidative mechanisms. When planning our study, our hypothesis was that the depression seen often in BD could further increase the oxidative damage in these patients [25, 26]. However, we observed no significant difference for the oxidative / antioxidative mechanisms between the BD group with depression and BD group without depression. The increased oxidative stress and deteriorated antioxidative mechanisms in BD therefore did not seem to be due to the depression frequently accompanying this disease. Indeed, FOR produced in excessive amounts by active neutrophils has been reported to cause oxidative tissue damage due to suppression of antioxidant defense mechanisms in the plasma, neutrophils, and erythrocytes and to be associated with the pathogenesis of the disease [27]. The oxidative/ antioxidative mechanisms can therefore be considered to have a role independent from the accompanying depression in the pathogenesis of BD. Although there was no difference between the BD group with depression and BD group without depression in terms of oxidative / antioxidative parameters, evaluating the correlation of the oxidative and antioxidative parameters and the HAM-D and HAM-A scores revealed a statistically significant positive correlation only between the MDA oxidant and HAM-D and HAM-A scores. There was no significant correlation between the antioxidants (TAC, PON, ARE, GSH) and the HAM-D and HAM-A scores. These findings suggest that depression and anxiety added to BD further increase the oxidative stress in this disease. Positive correlation between HAM-D and HAM-A with MDA oxidant, may indicate that oxidative stress is already increased in active and inactive BD. Addition of depression and anxiety to this disease may increase oxidative stress further. The presence of mild and moderate depression in BD patients in this study makes interpreting the results of the study difficult. Treatment of the depression accompanying BD can be suggested to decrease the progression of tissue damage due to oxidative stress that has an important role in the pathogenesis of the disease.In conclusion, our findings show that the oxidant/ antioxidant balance is disrupted in BD and oxidative stress is increased even in the inactive period. In addition, the deterioration in oxidative / antioxidative mechanisms in BD is not caused by the depression that often accompanies this disease. The oxidative / antioxidative balance has been reported to be disturbed in depression in the literature [9, 23, 24]. The oxidative / antioxidative balance that was already deteriorated in BD did not change with the addition of depression in our study. However, the generalization of our findings is limited due to a few factors. First, our study included a limited number of patients and it is difficult to say that they represent all patients with BD. Studying only the inactive period of BD patients was another limitation of our study. Our findings contribute to the information available on the pathogenesis and prognosis of the disorder while indicating the need for further studies. It will thus be possible to define the negative results of chronic disorders such as BD and provide a broader approach to the disorder during the therapeutic process.
ACKNOWLEDGEMENTS
- This study was supported by Inonu University Scientific Research Project Unit. The Authors declare that they have no conflict of interests.
Conflict of Interest
- The Authors declare that they have no conflict of interests
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML