Hanna Tahmasebi1, Reza Talebi2, Bahareh Rahmian Zarif3
1Department of Microbiology, Kurdistan Science and Research Branch, Islamic Azad University, sanandaj, Iran
2Department of Plant Breeding, College of Agriculture & Natural Resources, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
3Department of Biology, Sanandaj Branch, Islamic Azad University, sanandaj, Iran
Correspondence to: Bahareh Rahmian Zarif, Department of Biology, Sanandaj Branch, Islamic Azad University, sanandaj, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Background: Bacillus cereus is an opportunistic pathogen one of the major causing food poisoning, manifested by diarrhea or emetic syndrome. The present study undertaken to isolation B. cereus by Phenotype & Genotype; characterization of β-lactamase from B. cereus strain isolated from raw chicken meat. B. cereus produces a broad-spectrum β-lactamase and it is one of the potential Pathogenic reasons that makes the strains resistant, the blm β-lactamase is a gene that has the ability to resist. MaterialsandMethods: A total 250 sample collected from Breast, Tight, Wing, Liver and Gizzard of Raw Chicken from Sanandaj of Iran by PEMPA selective media, 39 B. cereus Isolated by 7, β-lactamse discs examined by antibiogram method, DNA extraction by CTAB and, the resistance strain checked for blm β-lactamse Resistance Gene by PCR. Result: The isolates of B. Cereus (15.6%) from chicken meat were highest resistance to Penicillin, Cloxacilin, Cephalexin (100%) and the least in Ampicillin (13%). 76.4% of the isolated Bacillus cereus has been blm. Discussion: This study examines the prevalence of B. cereus in chicken meat. The estimate that the poultry slaughterhouse contaminated with Bacillus cereus and will find its presence in the environment, can induce illness, The accessibility of appropriate protocols for treatment with β- lactamase antibiotics that comprise the bulk of antibiotics to use in clinics, poultry flocks and veterinary.
Keywords:
Bacillus Cereus, Chicken Meat, ß-lactamase, Resistance, blm, PCR
Cite this paper: Hanna Tahmasebi, Reza Talebi, Bahareh Rahmian Zarif, Isolated of Bacillus Cereus in Chicken Meat and Investigation β-Lactamase Antibiotic-Resistant in Bacillus Cereus from Chicken Meat, Advances in Life Sciences, Vol. 4 No. 4, 2014, pp. 200-206. doi: 10.5923/j.als.20140404.03.
1. Introduction
B. Cereus importance cause of economic, medical and biological defense [14]. This bacterium is an opportunistic type, is considered one of the causes of food poisoning. Its symptoms are watery diarrhea and vomiting associated with abdominal pain [6 ,7 ,8], Although improvement is achieved quickly, but rare reports of death due to the involvement of the various internal organs such as the heart, lungs, liver at necrosis [9] and fatal meningitis [10].The bacteria isolated from the environment, hospital, Variety of foodstuffs such as fish, meat, poultry, honey, vegetables, desserts and soups either in raw, cooked or processed mode [3, 4]. Bacillus cereus resistance to penicillin, cephalosporin and other β-lactam [12]. Chicken may be contaminated with Bacillus cereus during growth in poultry, that possible infected chickens or through contaminated poultry seed or hatchery. Equipment shows that the infection can be made cross contamination. To cause of several different aims, the antibiotics can be added to food and water of chicken. Low levels of certain antibiotics as growth stimulating are used and to fight special infections may be taken at a higher level. Under certain condition, numbers of antibiotics are added to feed for rapidly growth of chicken and increase it, improve feed efficiency or doing both of these. β-lactam antibiotics see among these [16], Which can be caused by beta-lactam resistance. As one of the important causes of antibiotic resistance in bacteria is Frequency increased of β-lactamases. [1] A wide range of β-lactam antibiotics include increasing bacterial resistance to antibiotics of this group it can be a cause of concern. [5]. β-Lactamase catalyzed hydrolysis of the amide bond in the β-lactam ring and thus inactivate the antibiotics. [15] The capability or incapability of a β-lactamase to resistance pertained on its kinetics, location and amount and on the physicochemical situation. The β-lactamase of gram-positive species are mainly extracellular, albeit, depending on the growth conditions, some enzyme might adhere to the cytoplasmic membrane. [12] One the reasons of β–lactam resistance is they have altered bacterial targets, penicillin-binding proteins (PBPs or transpeptidase) or Β-lactamases bind to the antibiotics and cleave the β-lactam ring. The antibiotic is no longer able to inhibit the function of PBP [11]. Bacillus cereus Gram-positive, rods, spore forming, Β-hemolysis positive and bacteria that can tolerate anaerobic and aerobic conditions. [2] blm ß-lactamase is mainly located in the chromosome of bacillus cereus. [17]ObjectivesThus, the present study investigated to incidence of B. cereus in chicken meat, analyzing by phenotypic test and verifying by molecular method, bal gene checked isolated strain. We use antibiograms; From the antibiograms, the numbers of isolates tested and the number of resistance isolated then to blm, β-lactamase resistance gene checked, As far as we know this is the first time the study has been done.
2. Materials and Methods
Bacillus cereus isolated from chicken meat samplesFor the isolation of Bacillus cereus, the foremost step is gathering samples of chicken meat. 250 samples randomly collected from poultry Slaughterhouse and meat markets of sanandaj in period of a year. (Kurdistan, Iran Northwest), as well from different part of chicken: Thigh, Breast, Wing, Heart, Liver, Gizzard were prepared. After collecting the samples, approximately 30 g of meat samples was separated and the special nylon zippers were transported to the laboratory in sterile conditions, proper temperature, and immediately were transferred to refrigerated 4℃ temperature under aseptic conditions. Initially samples in 90 ml BHIB, about 10 minutes on shaker, next of 24 hours, incubation at 32℃, final amount of specimen (10 ml supernatant) transferred the tube (The dilution in the tube would be 10-1), then tube transfer to bain-marie 65℃ for 15 minutes, after cooling tube, making serial dilutions until 10-3 Dilution. After 24 h was incubated in BHIB medium, a loop containing B. Cereus cultured on PEMBA (Polymyxin Pyruvate Egg Yolk Mannitol Bromothymol); selective medium of this bacteria and incubation at 37℃ for 48 h, after this period we observed blue colonies (peacock blue) with lecithinase zone and transferred it on nutrient agar for further study, Phenotypic tests; Gram staining, spore, catalase, β hemolysis, Starch, Nitrate reduction, Oxidase, motility, VP, OF. [13]Stoke For collecting and storing samples, we prepared stock containing Skim milk broth and glycerol; we’ve kept on Freezers - 20℃.When we want to use the next step, we cultured stocks again on blood agar.DNA ExtractionTotal DNA from B. Ceres was extracted by CTAB (cetyltrimethlyammonium bromide) method. Make suspension of bacterial colony (2 loop colony) by 700ml of CTAB buffer. (CTAB Buffer consisting: CTAB, EDTA, Polyvinyl pyrrolidone (PVP), NaCl, 2me, Tris-HCl) and Place the tube into 60°C water bath for 30 minute next step added with 450 μl of. Chloroform- isoamylalcohol (24:1 solution) and shake it, to have emulsion for 10 min in Room temperature and then centrifuged for 12000 rpm, 15 min. Transferred aqueous phase(top phase) to new tube and discard the chloroform phase to the new tubes was added the same volume of cold isopropanol and gently inverted until the DNA coil appears, first for an hour kept in the Freezer 20°C then in the refrigerator for 24 h, next we were centrifuged (12000 RPM, 15 min), the small DNA pellet was washed with 70% ethanol, Dried in desiccator in a very short period (20 min) and added in, 150 μl of distilled water. For the next steps we kept DNA in the freezer -20°C.Molecular AnalysisLater on isolation B .Cereus by phenotype test, in order to verify by PCR amplification method using specific primers for this bacteria name bal. The primer sequences for bal, given in the table below.Table 1. balF/balR primers
 |
| |
|
Primer “BalF – BalR” exclusive for Bacillus cereus group. (18)B.Cereus was isolated at this stage definitely.PCR reaction mixture mixture consisting of; 2.5 μl PCR buffer(1X), 1.5 μl MgCl2 (2 mM), 0.25 μl of dNTPs, 0.75 μl of each primer, 1 U Taq DNA polymerase (Sinna Gen, Iran) and 5 μl DNA template (3.33 ng per μl)1 with 25 μl final volume in each tube.Setup condition for DNA amplification is shown in table 3We were using Gel electrophoresis method. In DNA Amplification, explain more about it.Antibiotic susceptibility testingBacteria that were isolated and definitely were B. Cereus, Antibiogram Test was done for each one.The antibiotic discs and concentrations tested were as follows:Penicillin (10 µg⁄ Disc), Amoxicillin (25 µg⁄ Disc), Ampicillin (10 µg⁄ Disc), Ceftriaxone (5 µg⁄ Disc), Cloxcillin (30 µg⁄ Disc), Cephalexin (30 µg⁄ Disc).Antimicrobial Disk Diffusion Susceptibility (Bauer, Kirby) method used in this test, on Mueller-Hinton agar (CLSI, 2005).DNA Amplificationblm primers (resistance gene) were designed to amplify. (table 2)Table 2. blmF/blmR primers
 |
| |
|
PCR reaction mixture consisting of; 2.5 μl PCR buffer(1X)1, 3.75 μl MgCl2 (2 mM)1, 0.25 μl of dNTPs, 0.75 μl of each primer, 0.4 μl Taq DNA polymerase (SinnaGen, Iran) and 5 μl DNA template(3.33 ng per μl)1 with 25 μl final volume in each tube.The amplification conditions were as follows steps show in table 4.We were using Gel electrophoresis method, 1% agarose gel and Stained with ethidium bromide in TBE buffer and running at 95 V for 1 hour 30 min to controlled PCR products was present or not.Table 3. The PCR reaction with bal primer
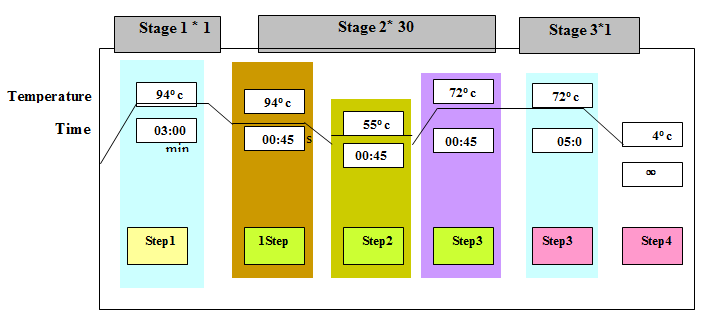 |
| |
|
Table 4. The PCR reaction with blm primer
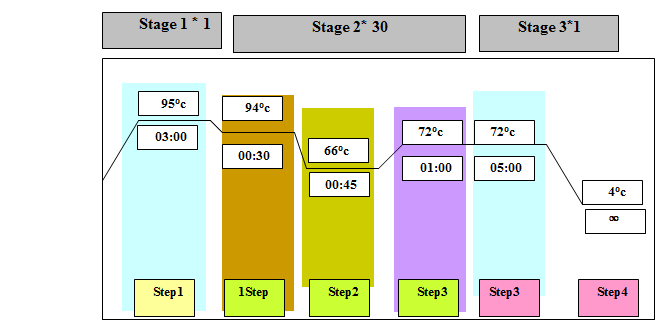 |
| |
|
3. Results
Isolation of B. cereus: A total of 250 Raw Chicken meat and Chicken offal samples from local Protein market and Poultry Slaughterhouse of Sanandaj (Table 5). Table 5. Presence of B. cereus in Raw Chicken meat and Chicken offal samples
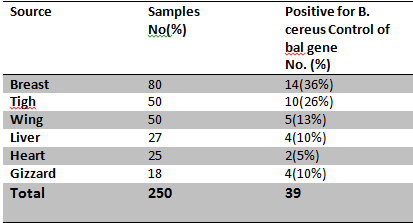 |
| |
|
At first by Morphological test, we were founded 46 positive B. Ceres.By morphological test showed Gram-positive, spore-forming, rod-shaped, motile, β-hemolysis and Starch positive.On PEMBA medium, observable peacock blue colonies with lecithinase zone.All 46 isolated showed performed based on the segmentation. (Fig 1)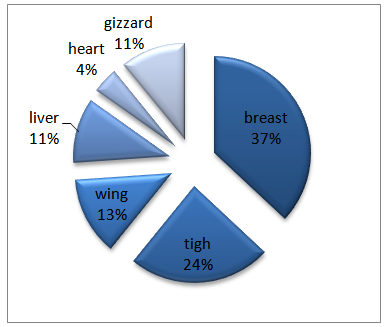 | Figure 1. Positive B. cereus by Morphological Test (%) |
Polymerase chain reaction first stage: checked for the presence of bal gene by B. cereus group specific primers BalF-BalR; 39 positive B. cereus were found. (Fig 2)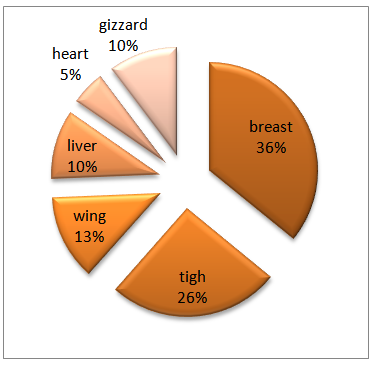 | Figure 2. Presence of B. cereus in Raw Chicken meat and Chicken offal samples by bal gene |
Was accompanied by 533 bp amplified product in 39 samples total 46 morphological isolated samples. (Fig 3)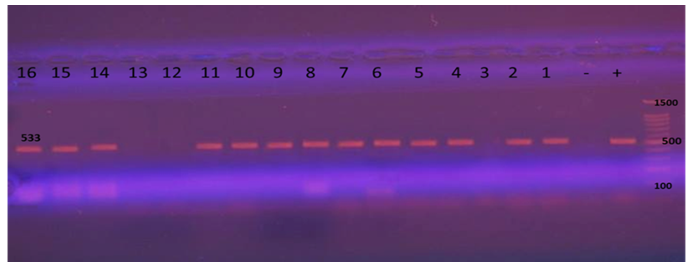 | Figure 3. B. cereus group specific (bal) PCR. Lane M: 100 bp DNA ladder |
Antibiotic susceptibility: β-Lactam susceptibility and resistance was performed on 39 of Bacillus cereus isolated. (table 6) The highest resistance was 100% and seen for Penicillin, Cloxacilin, Cephalexin and 13% showed Least resistance of Ampicillin. These antibiotics that suggests could be specific for isolated the blm gene.Table 6. Antibiotic resistance pattern of bacillus cereus isolates from chicken meat
 |
| |
|
Blm Amplification: After reviewing Bacillus cereus by antibiogram test results obtained stating that all 39 B. cereus were resistant to 3 or more antibiotics; as a result, all 39 B. cereus were investigated for the presence of resistance blm gene, yielded an amplified product of 2900 bp only in 30 isolates. (Fig 4)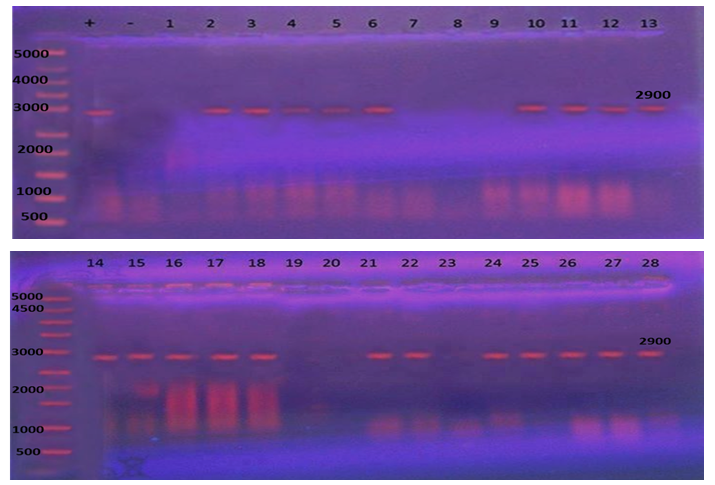 | Figure 4. β-lactamse gen resistant (blm) PCR. Lane M: 1000 bp DNA ladder |
Statistical analysis was performed by using SPSS/16.0 for a considerable relationship between resistance and district of Bacillus cereus isolated from meat and offal, there is not a significant relationship between blm-producing strains and chicken pieces Isolated. (P-value < 0.05)
4. Discussion
Bacillus cereus cause of the ability to food poisoning, resistant spores and extends in the environment, can easily cause infection. Due to improper preservation of food in some areas or Wrong transportation conditions, could be increases poisoning.It is noted that the increasing use of β-lactamase Antibiotics in poultry treatment of various forms; for example with food stuffs, mostly haven't Scientific the infrastructure and cause of increases the β-lactamase.Antibiotic resistance, transfer of gene resistance in bacteria and from bacteria to human in this way and varies other ways to mankind and the debasement of these valuable drugs are in medical treatment.This study was to find B. cereus Frequency in and blm β-lactam Resistance in B. cereus isolated from raw chicken.The main aim, understanding the correct use of antibiotics prescribed and correct diagnosis and treatment strategy in any area of the country.A study by J. Behravan and Z. Rangsaaz on Bacillus cereus 1015 was used to evaluate, the results of the antibiotic penicillin, Cloxcillin, Cephalexin, was the perfectly aligned [19], an another study from India closely similar to this study result for Amoxicillin, Ampicillin. [20]Blm gene was observed in 76.9% isolate of B. cereus, which could be one of the reasons for the β-lactam antibiotic resistance in this bacterium.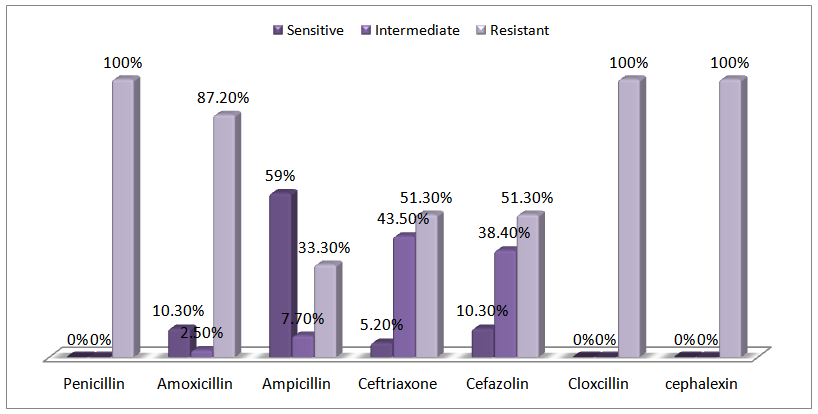 | Chart 1. Antibiotic susceptibility pattern of isolated B. cereus |
Because the Bacillus cereus is abundant in food such as chicken (a big portion of the food basket in the world covers), diagnosis through molecular method cause of its reliability and faster for the treatment and ward away from the antibiotic that their use is not necessary. We also supply this information in the veterinary organization and poultry slaughterhouse will be utilized to develop prevent to antibiotic resistance and the growth component of chicken and prevent disease in poultry flocks appropriate alternatives to be created.In the future, our goal is to study blm gene expression and other resistance; beta-lactamase gene resistance like bla1 and bla2 in chicken and other foodstuff and could study also MDR resistance in this bacterium.
ACKNOWLEDGEMENTS
The authors are thankful to Dr S. Amini for technical and moral support.
Note
1. The final concentration
References
| [1] | Majiduddin, F. K., I. C. Mateson, and T. G. Palzkill. 2002. Molecular analysis of β-lactamase structure and function. Int. J. Med. Microbiol. 292:127–137. |
| [2] | Washington CW Jr, Stephen D, William MJD, Gary W (2006). Koneman's Color Atlas and Textbook of Diagnostic Microbiology. Sixth Edition Lippincott wiiliams & wilkins. |
| [3] | Duc, L.H., T.C. Dong, N.A. Logan, A.D. Sutherland, J. Taylor and S.M. Cutting, 2005. Cases of emesis associated with bacterial contamination of an infant breakfast cereal product. Int. J. Food Microbiol., 102: 245-251. |
| [4] | Svensson, B., A. Monthan, M. Guinebretiere, C. Nguyen-The and A. Christiansson, 2007. Toxin production potential and the detection of toxin genes among strains of Bacillus cereus group isolated along the dairy production chain. Int. Dairy J., 17: 1201-1208. |
| [5] | Medeiros, A.A. (1997) Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24, S19–45. |
| [6] | Schoeni JL, Wong AC. Bacillus cereus food poisoning and its toxins. J Food Prot 2005; 68: 636-48. |
| [7] | Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, et al. Fatal family outbreak of Bacillus cereus - associated food poisoning. J Clin Microbiol 2005; 43: 4277-9. |
| [8] | Batt C.A. (1998): A risk assessment approach for foodborne Bacillus cereus and its toxins. Journal of Applied Microbiology (Symposium Supplement), 84: 51–61. |
| [9] | Dirnhofer R, Sonnabend 0, Sonnabend W 1977 Eine tiidlich verlaufene lebensmittelvergiftung durch Bacillus cereus. Zeitschrift fur Rechtsmedizin 80 : 139-1 5 1. |
| [10] | Evreux F, Delaporte B, Leret N, Buffet-Janvresse C, Morel A. A case of fatal neonatal Bacillus cereus meningitis. Arch Pediatr 2007; 14: 365-8. |
| [11] | Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995, 8(4):557. |
| [12] | Myers, J.L. and Shaw, R.W. (1989) Production, purification and spectral properties of metal dependent β-lactamases of Bacillus cereus. Biochim. Biophys. Acta 995, 264–272. |
| [13] | Gordon, R. E. W. C., Haynes, C. H.-N,. Pang. The genu Bacillus. Handbook, No. 427. U.S. Department of Agriculture. Washington, D.C. 1973. |
| [14] | Sakai H, Howlader M, Ishida Y, Nadaguci A, Oka K, Ohbayashi K. Replacement of domain lll of cry insecticidal proteins from Bacillus thuringiensis. Journal of bioscience and bioengineering. 2007; 103: 381 -3. |
| [15] | Ambler RP .The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci.1980; 289:321–331. |
| [16] | Fatemi A, and Mirzaey S. Drug treatment of ornamental birds. tehran: Publications Nourbakhsh. |
| [17] | Barry G, Hall, Stephen J. Salipante, Miriam Barlow. The Metallo-b-Lactamases Fall into Two Distinct Phylogenetic Groups. Journal of MOLECULAR EVOLUTIN. 2003; 57: 249–254. |
| [18] | Chang YH, Shangkuan YH, Lin HC, Liu HW. PCR assay of the groEL gene for detection and differentiation of Bacillus cereus group cells. Appl Environ Microbiol 2003; 69: 4502-10. |
| [19] | J Behravan J, Rangsaaz Z. Detection and Characterization of β-Lactam Resistance in Bacillus cereus PTCC 1015. The Scientific World JOURNAL. 2004; 4, 622–627. |
| [20] | Rather, M.A., Aulakh, R.S., Gill, J.P.S., Ghatak, S. Enterotoxin gene profile and antibiogram of bacillus cereus strains isolated from raw meats and meat products , Journal of Food Safety .2011 ; ISSN 1745-4565. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




