-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(3): 156-165
doi:10.5923/j.als.20140403.11
Probiotic Antagonism of Sphingomonas sp. against Vibrio anguillarum Exposed Labeo rohita Fingerlings
Asma Chaudhary1, Javed Iqbal Qazi2
1Division of Science and Technology, University of Education, Lahore, 54770, Pakistan
2Environmental Microbiology Laboratory, Department of Zoology, University of the Punjab, Lahore, 54590, Pakistan
Correspondence to: Asma Chaudhary, Division of Science and Technology, University of Education, Lahore, 54770, Pakistan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Antagonistic properties of Sphingomonas sp. AsCh-P3 against fish pathogen V. anguillarum was investigated in vitro and in vivoassays. Out of twenty probiotic isolates, nine expressed antagonistic activity by cross streak and six gave prominent results by disc and well diffusion methods. Maximum growth inhibition zone (15 mm) was observed by Sphingomonas sp. AsCh-P3. Cross streaks of six isolates of known C.F.U./ml across different concentrations of pathogen indicated profound growth inhibition by low concentration. The six bacterial isolates were mixed in fish feed, dried and stored in refrigerator upto 4 days. Sphingomonas sp. AsCh-P3 showed 63.5% survival in dried feed under the storage condition and grew best with 30% inoculum size in 20% fish feed extract. The Labeo rohita fingerlings were exposed to V. anguillarum intraperitoneally. Fish mortalities appeared in a dose dependant manner. Pathogen exposed fishes were provided with Sphingomonas sp. AsCh-P3 treated feed in different experimental groups as G1-soon after, G2- prior and after and G3- prior challenge. Percent mortalities of 33.33, 16.66 and 23.33 and RPS values of 16.67, 58.35 and 41.67 were recorded for G1, G2, and G3, respectively. Probiotic loads reduced the pathogens virulence in dose responsive manners.
Keywords: Probiotics and fish health, Antagonistic bacteria to fish pathogen, Virulence, Vibrio anguillarum, Disease resistance, Sphingomonas, Vibriosis, Labeo rohita
Cite this paper: Asma Chaudhary, Javed Iqbal Qazi, Probiotic Antagonism of Sphingomonas sp. against Vibrio anguillarum Exposed Labeo rohita Fingerlings, Advances in Life Sciences, Vol. 4 No. 3, 2014, pp. 156-165. doi: 10.5923/j.als.20140403.11.
Article Outline
1. Introduction
- Intensive quaculture has led to higher outbreaks of disease encompassing an ever increasing range of pathogens [1, 2]. Efforts have been made to reduce infections caused by Vibrio, this genus has been associated with severe disease in fish culture [3, 4]. Species such as Vibrio harveyi, V. anguillarum, V. alginolyticus, V. parahaemolyticus and V. Vulnificus have frequently been associated with mortalities both in hatcheries and grow out ponds [5-7].Vibriosis occurs predominantly in marine and brackish waters and occasionally in fresh water fish species. V. anguillarum is the most commonly encountered fish pathogen [8-10]. V. anguillarum is a Gram negative bacterium that causes hemorrhagic septicemia in fish, a disease that leads to great economic losses in fish farming worldwide. Although this bacterium has been reclassified as Listonella anguillarum based on 5S rRNA gene sequence analysis, however, it is still commonly referred to as V. anguillarum [11].Prevention of fish diseases by the application of live pathogen-antagonistic bacteria has received widespread interest and such probiotics are gaining much popularity [12-16]. Probiotics refer to beneficial (live) bacterial cultures whose applications improve health of the host. A probiotic must not be pathogenic or toxic to its host. Most probiotics are supplied as live supplements in food and they obviously have the ability to survive in intestinal tract where they break down toxic or innutritious components of the diet and/or may prevent potential pathogens from colonizing the gut by production of antimicrobial compounds, or by out competing them for nutrients or mucosal space [17-20]. In this work, first virulance of V. anguillarum to healthy Labeo rohita fingerlings has been established. Purpose of the present study was to determine antipathogenic potential of Sphingomonas sp. AsCh-P3 in L. rohita fingerlings following the vibriosis trial.
2. Materials and Methods
2.1. Source of Fish Pathogens
- Virulent strain of Vibrio anguillarum (90-11-287; sero type 01) that carries the pJM1 plasmid was a generous gift from K. Pedersen, Royal Veterinary and Agricultural University, Copenhagen, Denmark [21].
2.2. Isolation and Selection of Probiotic Bacterial Isolates
- Twenty bacterial isolates were secured from different home made yogurt and raw milk samples for studying their antagonistic activity against the two fish pathogens [22].
2.3. Inhibition of Fish Pathogens by the Bacterial Isolates
- Antagonism of V. anguillarum by the bacterial isolates was assessed by cross streaking method [23]. For this purpose, suspension of the pathogen in phosphate buffer saline (O.D 0.5±0.05) was streaked at right angles across streaked inocula of the bacterial isolates (O.D. 0.5±0.05) to be tested on nutrient agar. The plates were incubated at room temperature (~27-30°C) for 24-48 hours. Antagonism was indicated by an interruption in the growth of the pathogens. From the twenty bacterial isolates tested, nine isolates were selected on the basis of their prominent antibiosis against the pathogen for further validation through filter paper disc and well diffusion methods.
2.4. Antagonism Assay
- Antagonistic activity of the nine select bacterial isolates against V. anguillarum was assessed by disc and well diffusions methods [24]. The pathogen and the probiotics were grown in nutrient broth for an overnight period at 37ºC, centrifuged and filter sterilized. One hundred micro liters of each filtered sterilized culture fluid was loaded on 9 mm diameter sterile disc made from Whatman filter paper No. 1. These discs were placed on nutrient agar plates seeded with 50 µl of V. anguillarum suspension of 0.5±0.05 O.D. The plates were incubated at room temperature (~27-30ºC) for 24-48 hours and the zones of growth inhibition around the discs were then measured. For well diffusion method, V. anguillarum inoculums was mixed in nutrient agar medium (kept molten around 50ºC) at 3% (v/v) ratio and poured in sterilized petri plates. Wells of 9 mm diameter were punched into the solidified agar with the help of a metal borer and then 100 µl of filtered sterilized culture fluid of a given bacterial isolate was introduced in a well. The plates were incubated at room temperature (~27-30ºC) for 24-48 hours and growth inhibition zones (GIZ) were then recorded. The five bacterial isolates which yielded GIZ of higher than 11 mm diameter were selected and proceeded for further study.
2.5. Inhibition of the Probiotics against Different Concentrations of the Fish Pathogen
- To find antibiosis of the probiotics against different concentrations of the pathogen, cell suspensions of 0.05, 0.1, 0.25, 0.5, and 1.0 O.D. at 600 nm were prepared in phosphate buffer saline (PBS) of pH 8. Cell densities of the probiotics were adjusted at 0.5±0.05 in the buffer. Viable counting of the bacterial suspensions was performed on nutrient agar. The suspensions of the bacterial isolates were streaked at right angles across streaked inocula representing different concentrations of the pathogen on nutrient agar. The plates were incubated at room temperature (~27-30ºC) for 24-48 hours. Interruption and its intensity in the growth of the pathogen indicated the level of antibiosis of test bacterial isolates [23].
2.6. Inoculation of Probiotics Isolates in Fish Feed
- The select bacterial isolates were grown overnight in nutrient broth, centrifuged and the pellets suspended in PBS up to cell density of 0.5 ± 0.05 at 600 nm. The bacterial suspensions (20% v/w) were mixed with autoclaved formulated fish feed and dried in a laminar flow cabinet, kept at room temperature (~27-30ºC) for three days and then freezed for one week period. Serial dilutions of the processedvfeed samples were prepared in saline water (0.89%) and analyzed for viable counting on nutrient agar plates. The percent survival of each of the bacterial isolate was calculated as follows; % survival = (Ao/A1) × 100. Where; Ao = C.F.U. of bacteria per gram after drying and A1 = C.F.U. of bacteria per gram before drying. On the basis of the antagonistic and survival potentials, the probiotic isolate later identified as Sphingomonas sp. AsCh-P3 was selected for inoculum optimization in the formulated fish feed.
2.7. Optimizing Bacterial Inoculum for the Fish Feed
- For the inoculum optimization, instead of solid feed, its extract was used. For this purpose, 20 g of the feed were boiled in 100 ml distilled water for 10 minutes and then autoclaved, routinely, for 15 minutes. The autoclaved feed was centrifuged to get clear extract. Cell suspension of the bacterial isolate, AsCh-P3 in PBS was inoculated at 10%, 20% and 30% (v/v) in the feed extract. The cultured feed extracts were incubated at room temperature (30±0.2oC) and the growth was assessed daily up to 3 days post incubation as C.F.U./ml.
2.8. Identification and Biochemical Characterization of the Bacterial Isoltes
- For taxonomic identification, all the bacterial isolates were subjected to colonial, cell morphology and biochemical tests [25-27] and identified accordingly. However, identification of the select strain AsCh-P3 screened through its 16S rRNA gene sequencing.
2.9. DNA Extraction, 16 S rRNA Gene Amplification and Sequencing
- Total DNA of the isolate was extracted from its fresh culture. A loopful of the culture from a colony on nutrient agar was heated in water bath (95ºC) in 45µl of 50 mM NaOH followed by addition of 5 µl of 1M Tris HCl (pH 8) and the eppendorff was centrifuged for 10 minutes. Amplification of the 16S rRNA gene was then accomplished by PCR with DNA polymerase (KOD FX). The reaction mixture comprised of 2 mM deoxynucleoside triphosphates (dNTP), 50 uM of each 27F(5-AGAGTTTGATCCTGGCTCAG-3) and 1492R (5-AGGCTACCTTGTTACGACTT-3) primers and 2 µl of the template DNA (bacterial supernatent). PCR was performed for 30 cycles, each consisting of a 10-sec denaturation step at 98℃, a 30-sec annealing step at 53℃ and a 1-min extension step at 72℃. PCR product was purified and partially sequenced on automated sequencer.The sequence was subjected to homology search using BLAST-querying the GenBank database. http://www.ncbi.nlm.nih.gov/blast (last accessed March, 2011) of the National Center for Biotechnology Information (NCBI).
2.10. Pathogenecity of Vibrio anguillarum in Labeo rohita Fingerlings
- Labeo rohita fingerlings were obtained from the fish farm at Muridkey in the month of August. They were acclimated to the experimental conditions for 10 days in the laboratory. Health status of the fish was kept under examination throughout the acclimatization period.The fingerlings were initially exposed to Vibrio anguillarum in bath challenge but neither disease symptoms nor mortality were observed. Therefore, intraperitoneal (i.p) inoculation was accomplished according to the methods described by Romalde et al. [28] and Robertson et al. [29]. Accordingly, overnight fresh culture of Vibrio anguillarum was centrifuged, suspended in PBS at cell densities of 1.0, 0.5, 0.25, 0.1, 0.05 which were marked out for C.F.U./ml as 50 ×109, 243 ×105, 56×105, 267×103, 97×103 respectively. To examine the virulence of the fish pathogen, groups of 10 Labeo rohita fingerlings received i.p injections of different concentrations of the bacterium. All fishes in each group were injected intraperitoneally with 0.1 ml of PBS suspension representing a given concentration of fish pathogen. Control group was injected with sterile PBS in the same manner. Each group was housed in a separate aquarium at 27-30ºC containing 10 liter static fresh water and continuously aerated through an air stone. One third water of aquarium was daily changed and the feces were siphoned off. The fish groups were monitored up to 30 days and fed sterilized formulated fish feed. Mortalities were recorded daily. Pathogen dose of 56×105C.F.U./ml with 40% mortality was selected for next experiment.
2.11. Probiotic Treatment of Infected Fish
- The bacterial isolate Sphingomonas sp. AsCh-P3 showing higher antagonistic activity for V. anguillarum in vitro assay was employed for the in vivo study. These experiments were conducted after consulting the methods described by Gram et al. [30] and Spanggaard et al. [31]. For in vivo experiment, the experimental set up is described in the table 1.
 | Table 1. Details of the experimental set up for probiotic application to the infected fish |
2.12. Statistical Analysis
- All the experimental data were analyzed using one way analysis of variance (ANOVA) followed by Tukey’s multiple range test (SPSS ver.16.0 software, SPSS, Chicago, IL, USA).
3. Results
3.1. In Vitro Bacterial Antagonism against the Fish Pathogen Vibrio anguillarum
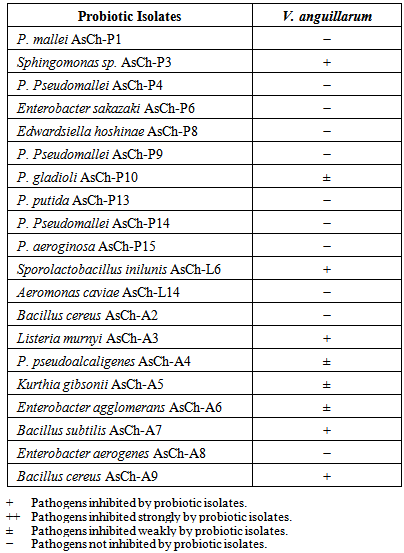
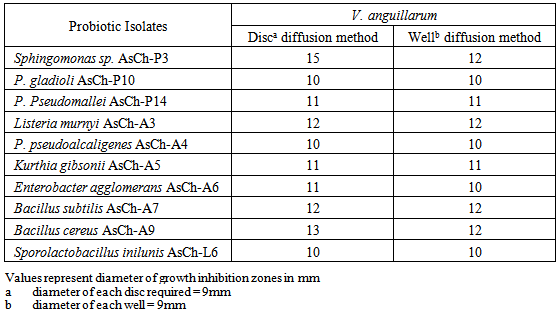
- Twenty bacterial isolates were tested for their antibiosis against the fish pathogen Vibrio anguillarum by cross streak method (Table 2). As can be seen from this Table, only 09 bacterial isolates expressed antagonism against the pathogen, while 11 could not exert any growth inhibiting effect to the pathogen. Based upon the growth inhibition zones of the cell free by filtered sterile broths of the probiotic isolates were selected for further study. Sphingomonas sp. AsCh-P3 showed highest GIZ of 15 mm against the pathogen whereas the remaining select isolates showed GIZ in the range 11-13 mm against the pathogen (Table 3).
|
|
3.2. Growth Inhibition of the Select Probiotic Bacterial Isolates against Different Concentrations of the Fish Pathogen
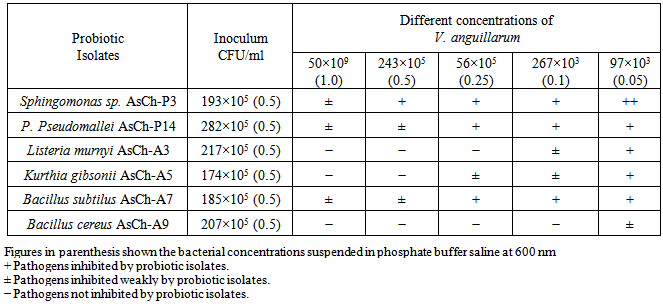
- Six probiotic isolates of known C.F.U./ml were streaked across inocula of strength of the fish pathogen (Table 4). Best antibiosis was shown by Sphingomonas sp. AsCh-P3 against V. anguillarum at all concentrations. However, a general trend indicated profound growth inhibition for the pathogen streaked with inocula of test organisms containing less number of C.F.U./ml.
|
3.3. Viable Counts of the Probiotics in the Formulated Feed
- Provision of food loaded with probiotic to an animal is generally characterized by an intervening period required for transporting and storage etc. This experiment was designed to observe the viability of the probiotic in the formulated feed, followed by drying at room temperature for 3 days and then storing in refrigerator for 4 days (Table 5, Figure 1). The select bacterial isolate Sphingomonas sp. AsCh-P3 showed highest survival rate up to 63.5% in the feed (Table 6). The bacterium grew best at 30% inoculum at 27-30ºC (Table 7, Figure 2). On the basis of results of antagonistic experiments and best survival rate in fish feed, Sphingomonas sp. AsCh-P3 was selected for in vivo study.
 | Figure 1. Viable counts of the selected probiotic bacterial isolates in fish feed during drying at room temperature (day 1-3) and storage in refrigerator (upto day 7) |
|
|
 | Figure 2. Effect of inocula sizes on the growth of the probiotic isolate Sphingomonas sp. AsCh-P3 in fish feed extract (20% w/v) for different incubation days |
3.4. Characterization and Identification of AsCh-P3 Isolate
- Colonies of bacterial isolate AsCh-P3 on nutrient agar appeared irregular, raised, creamy white with wavy margins, opaque and butyreus in consistency and measured up to 2.5 mm in diameter.Bacterial isolate AsCh-P3 was Gram-negative slender rod shaped cells with rounded ends measured up to 1.5×1 µm. The facultative anaerobe was motile by means of 2 polar flagella. The isolate could not require sodium chloride for growth but tolerated up to 6% salt concentration grew at 25, 37 and 45ºC, reduced nitrate with no gas production. The urease negative bacterium yielded positive results for catalase, oxidase, Voges Proskauer, and methyl red tests. The strain showed good growth at MacConkey agar, no growth at cetrimide agar.On the basis of morphological, biochemical tests and 16S rRNA gene sequencing, the isolate AsCh-P3 exhibited 99% similarity to 16S rRNA gene sequence of Sphingomonas sp.
3.5. Pathogenecity of V. anguillarum and Effects of the Probiotic Isolate
- Mortalities in Labeo rohita fingerlings infected with V. anguillarum, mortalities appeared in a dose dependant manner (Table 8). The pathogen dose which resulted into 40% mortality was selected for further investigation. The fishes exposed to V. anguillarum showed symptoms of reddening of body, swelling and reddening of belly, hemorrhage and erythma in eyes (Figure 3).
 | Figure 3. Typical vibriosis symptoms i-e reddening of body (a), swelling of belly (b), hemorrage/erythma in eye (c) and reddening of belly (d). following i-p administration of V. anguillarum (56×105 C.F.U/ml) |
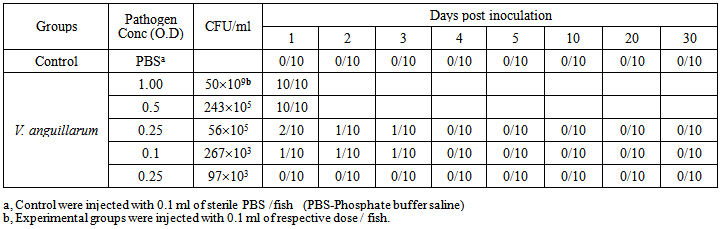 | Table 8. Mortality of L. rohita fingerlings within one month challenged by the pathogen V. anguillarum administered intraperitoneally |
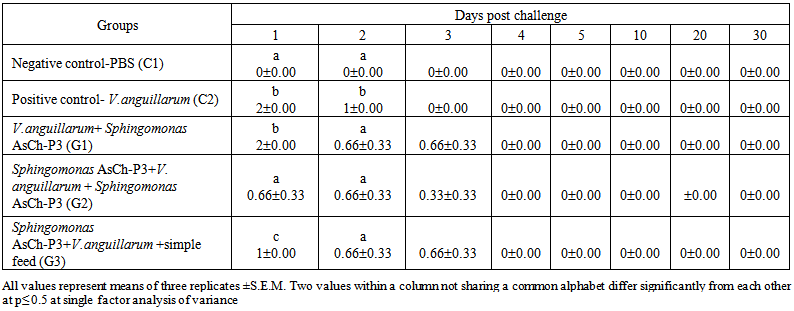 | Table 9. Mortality of L. rohita fingerlings within one month challenged by the fish pathogen V. anguillarum administered intraperitoneally and fed with Sphingomonas sp. AsCh-P3 augmented feed at 3% wet body weight under different experimental conditions |
 | Table 10. Relative percent survival within one month experimental period of L. rohita fingerlings challenged with V. anguillarum intraperitoneally and fed with Sphingomonas sp. AsCh-P3 augmented feed |
4. Discussion
- Nine out of the twenty bacterial isolates tested by cross streak method against the fish pathogen V. anguillarum showed positive results. Filtered sterilized supernatants of the isolates also manifested inhibitory effects on growth of the pathogen. This indicated that exo-products of the probiotics are involved in controlling the pathogen. It is well known that cell free supernatants of probiotic bacteria exert inhibitory activity against pathogens in aquaculture system with varying degrees of stability [30, 32-35]. It is also known that microbial populations may release chemical substances that have a bactericidal or bacteriostatic effect on other microbes. Production of antibiotics [36], bacteriocins [37], siderophores [38], lysozymes, proteases, and/or hydrogen peroxide and the alteration of pH values by the production of organic acids [39] are some of the most familiar processes which alter inter population relationships by influencing the outcome of competition for chemicals or available energy [38, 40] and show wide range of mechanisms underlying the antagonistic effects of probiotics. Probiotic should be ingested in high enough numbers to beneficially affect the host’s health. Good viability of probiotics in fish feed before its consumption is required for achieving their positive roles. Fish feed is usually formulated with a low water activity to prevent microbial deterioration over several months. This practice allows achieving a high stability during the drying process, with final moisture content closer to the original value of the unprocessed feed [41, 42]. Vibrio anguillarum, a marine fish pathogen, could show host specificity when was inoculated intraperitoneally (i-p) in Labeo rohita fingerlings. The fresh water fish was susceptible to vibriosis and majority of the dead fingerlings showed hemorrhages of intestine, swelling of belly and reddening of ventral part of the body. Similar symptoms had been reported previously in flat fishes suffering from vibriosis [3, 43-45]. It was found that the mortalities were dependent on dose of the bacterial pathogen. Virulence of V. anguillarum became evident with a final mortality of 40% in fish challenged with 56×105 C.F.U. /ml. Hjelm et al. [46] reported 80% mortality in turbot expressed to V. anguillarum at concentration ranging from 103 to 107 C.F.U. /ml.There are several hypotheses about the routes of entry of V. anguillarum in fish; oral delivery of the pathogen by live food has been reported in turbot post larvae [47, 48]. Skin, gills and anus are also important portals of entry of V. anguillarum into eel [49], rainbow trout [50] and ayu [51] respectively. Olsson et al. [52] proposed that Vibrio cells penetrate the intestinal mucus but epithelial cell penetration or endocytosis was not evident. However, bacterial infections are not uncommon in aquaculture. Mortalities in groups fed with the probiotic supplemented feed only after pathogen administration turned out to be higher than the group which was fed probiotics containing feed both before and after the administration. This indicates that the probiotic might have been colonizing the host but they might had required further probiotic cells and/or time for increasing their antipathogenic potential. It may be speculated that the presumed colonization of probiotic earlier to the pathogen challenge might have prevented growth and virulence of the pathogen. Reduced percent mortality and increased RPS have been reported by medicinal plants extract supplemented fish feed [7, 53]. Conclusively, the probiotic Sphingomonas AsCh-P3 supplemented feed significantly decreased the fish mortalities following the exposure to the V. anguillarum under different experimental conditions as compared to positive control in Labeo rohita fingerlings. These results necessitate the provision of healthy microbial communities in aquaculture systems.
5. Conclusions
- The study is an evaluation of inhibitory effects of bacterial strain Sphingomonas sp. AsCh-P3 against fish pathogen Vibrio anguillarum which appeared as a good probiotic candidate and will be used as additive in fish feed in future.
ACKNOWLEDGEMENTS
- This study is a part of author’s Ph.D work and is grateful to Punjab University for financial support and Dr. J.I. Qazi for his guidance.
References
| [1] | B. Austin and D. A. Austin, Bacterial fish pathogens; Disease of farmed and wild fish, 3rd (revised) ed., Godalming: Springer-Praxis, 1999. |
| [2] | Seenivasan, C., Saravana Bhavan, P., Radhakrishnan, S., and Muralisankar, T., 2012, Effects of probiotics on survival, growth and biochemical constituents of freshwater prawn Macrobrachium rosenbergii post larvae, Turkish Journal of Fisheries and Aquatic Sciences, 12, 331–338. |
| [3] | Diggles, B. K., Carson, J., Hine, P. M., Hickman, R. W., and Tait, M. J., 2000, Vibrio Species associated with mortalities in hatchery-reared turbot (Colistium nudipinnis) and brill (C. guntheri) in New Zealand, Aquaculture, 183, 1–12. |
| [4] | Estes, R. M., Friedman, C. S., Elston, R. A., and Herwig, R. P., 2004, Pathogenicity testing of shellfish hatchery bacterial isolates on Pacific oyster Crassostrea gigaslarvae, Dis. Aquat. Organ., 58, 223–230. |
| [5] | Mohney, L. L., Lightner, D. V., Bell, T. A., 1994. An epizootic of vibriosis in Ecuadorian pond reared Penaeus vannamei Boone (Crustacea:Decapoda). J. World Aquaculture Soc., 25(1), 116–125. |
| [6] | Zhou, L., Wang, X., Liu, Q., Wang, Q., Zhao, Y., Zhang, Y. X., 2010. A novel multivalent vaccine based on secretary antigen-delivery induces protective immunity against Vibrio anguillarum and Aeromonas hydrophila. J. Biotechnol., 146, 25–30. |
| [7] | Bilen, S., Yılmaz, S., Bilen, A. M., 2013. Influence of tetra (Cotinus coggygria) extract against Vibrio Anguillarum infection in Koi Carp, Cyprinus carpio with reference to haematological and immunological changes. Turkish Journal of Fisheries and Aquatic Sciences, 13, 517–522. DOI: 10.4194/1303-2712-v13_3_16. |
| [8] | Schreckenbach, K., 1974. Active immunization of fishes against Vibrio anguillarum. Z. Binnenfischerei DDR, 21, 167–172. |
| [9] | Ghittino, P., Andruetto, S., Vigliani, E., 1975. A serious vibriosis in rainbow trout grown in salt water. Riv. Ital. Piscicolt. Ittiopatol., 10(4), 113–115. |
| [10] | A. E. Ellis, General principles of fish vaccination. In: Ellis AE, Ed. Fish vaccination. London, Academic Press, pp. 1–19, 1988. |
| [11] | MacDonell, M. T. and Colwell, R. R., 1984. Nucleotide base sequence of vibrionaceae 5 S rRNA. FEBS Lett., 175(1), 183–188. |
| [12] | Vine, N. G., Leukes, W. D., Kaiser, H., 2004. In vitro growth characteristics of five candidate aquaculture probiotics and two fish pathogens grown in fish intestinal mucus. FEMS Microbiology Letters, 231(1), 145–152. |
| [13] | Dhanasekaran, D., Subhasish Saha, Thajuddin, N., Panneerselvam, A., 2008. Probiotic effect of Lactobacillus isolates against bacterial pathogens in Clarias orientalis. Facta Universitatis Series: Medicine and Biology, 15(3), 97–102. |
| [14] | Authira, R. R., Kirithika, M., Venkatesan, S., Ganesan, R., Muthuchelian, K., 2011. Studies on in vivo and in vitro antagonistic activity of probiotics against fish pathogens. Journal for Bloomers of Research, 4(1), 271–275. |
| [15] | Lara-Flores, M., 2011. The use of probiotic in aquaculture an overview. International Research Journal of Microbiology, 2(12), 471–478. |
| [16] | Tabak, S., Maghnia, D., Bensoltane, A., 2012. The antagonistic activity of the lactic acid bacteria (Streptococcus thermophilus, Bifidobacterium bifidum and Lactobacillus bulgaricus) against Helicobacter pylori responsible for the gastroduodenals diseases. Journal of Agricultural Science and Technology A, 2, 709–715. |
| [17] | R. Fuller, History and development of probiotics. In: R. Fuller, Ed., Probiotics. The scientific basis. New York. N.Y: Chapman and Hall, pp. 1–8, 1992. |
| [18] | Smoragiewicz, W., Bielecka, M., Babuchawowski, A., Boulard, A., Dubeau, H., 1993, Les probiotiques, Can. J. Microbiol., 39, 1089–1095. |
| [19] | Irianto, A., and Austin, B., 2002, Probiotics in aquaculture. J. Fish Dis., 25(11), 633–642. |
| [20] | Gobinath, J., and Ramanibai, R., 2012, Effect of probiotic bacteria culture on pathogenic bacteria from fresh water fish Oreochromis mosambicuss, J. Modern Biotechnology, 1(1), 50–54. |
| [21] | Skov, M. N., Pedersen, K., Larsen, J. L., 1995, Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1, Appl. Environ. Microbiol., 61,1540–1545. |
| [22] | A. Chaudhary, “Growth promoting and bacterial infections controlling roles of probiotics in rohu (Labeo rohita) fingerlings, Ph. D thesis, University of the Punjab, Lahore, Pakistan, 2007. |
| [23] | Austin, B., Billand, E., Stobie, M., 1992, Inhibition of bacterial fish pathogens by Tetraselmis succica, J. Fish Dis., 15, 55–61. |
| [24] | Olsson, J. C., Westerdahl, A., Conway, P. L., Kjelleberg, S., 1992, Intestinal colonization potential of Turbot (Scophthalmus maximum) and Dab (Limanda limanda) associated bacteria with inhibitory effects against Vibrio anguillarum, Appl. Environ. Microbiol., 58, 551–556. |
| [25] | S. R. Weyant, C. Wayne, E. R. Moss, G. D. Weaver, D. Hollis, J. Jordan, C. E. Cook, Identification of unusual pathogenic Gram-negative Aerobic and Facultative anaerobic bacteria, London: Williams and Wilkins, 1984. |
| [26] | H. J. Benson, Microbiological applications. Dubuque, USA: Wm. C. Brown Publishers, 1994. |
| [27] | C. H. Collins, P. M. Lyne, J. M. Grange, Colllins and Lyne’s microbiological methods. Oxford: Butterworth-Heinemann Ltd, 1995. |
| [28] | Romalde, J. L., Magarinos, B., Nunez, S., Barja, J. L., Taranzo, A. E., 1996, Host range susceptibility of Enterococcus sp. Strouns isolated from diseased Turbot; Possible routes of infection, Appl. Environ. Microbiol., 62, 606–611. |
| [29] | Robertson, P. A. W., O’Dowd, C., Burrells, C., Williams, P., Austin, B., 2000, Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss Walbaum), Aquaculture, 185, 235–243. |
| [30] | Gram, L., Melchiorsen, J., Spanggaard, B., Huber, I., Nielsen, T. F., 1999, Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible priobiotic treatment of fish, Appl. Environ. Microbiol., 65, 969–973. |
| [31] | Spanggaard, B., Huber, I., Nielsen, J., Sick, E. B., Pipper, C. B., Martinussen, T., Slienrendirecht, W., Gram, L., 2001, The probiotic potential against vibriosis of the indigenous microflora of rainbow trout, Environ. Microbiol., 3, 755–765. |
| [32] | Guerinot, M. L., 1994, Microbial Iron Transport, Annu. Rev. Microbiol., 48, 743–772. |
| [33] | Austin, B., Stuckey, L. F., Robertson, P. A. W., Effendi, I., Griffith, D. R. W., 1995, A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, Vibrio anguillarum and Vibrio ordalii, J. Fish Dis., 18, 93–96, doi: 10.1111/j.1365-2761. 1995.tb01271.x. |
| [34] | Rengpipat, S., Phianphak, W., Piyatiratitivorakul, S., Menasveta, P., 1998, Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth, Aquaculture, 167, 301–313. |
| [35] | Gram, L., Lovold, T., Nielsen, J., Melchiorsen, J., Spanggaard, B., 2001, In vitro antagonism of the probiont Pseudomonas fluorescens strain AH2 against Aeromonas salmonicida does not confer protection of salmon against furunculosis, Aquaculture, 199, 1–11. |
| [36] | Williams, S. T., and Vickers, J. C., 1986, The ecology of antibiotic production, Microb. Ecol., 12, 43–52. |
| [37] | Bruno, M. E. C., and Montville, T. J., 1993, Common mechanistic action of bacteriocins from lactic acid bacteria, Appl. Environ. Microbiol., 59, 3003–3010. |
| [38] | Pybus, V., Loutit, M. W., Lamont, I. L., Tagg, J. R., 1994, Growth inhibition of the salmon pathogen Vibrio ordalii by a siderophore produced by Vibrio anguillarum strain VL4335, J. Fish Dis., 17, 311–324. |
| [39] | Sugita, H., Shibuya, K., Hanada, H., Deguchi, Y., 1997, Antibacterial abilities of intestinal microflora of the river fish, Fisheries Sci., 63, 378–383. |
| [40] | Lemos, M. L., Dopazo, C. P., Toranzo, A. E., Barja, J. L., 1991, Competitive dominance of antibiotic-producing marine bacteria in mixed cultures, J. Appl. Bacteriol., 71, 228–232. |
| [41] | Poolman, B., and Glaasker, E., 1998, Regulation of compatible solute accumulation in bacteria, Molecular Microbiology, 29, 397–407. |
| [42] | Delcour, J., Ferrain, J., Deghorain, M., Palumbo, E., Hols, P., 1999, The biosynthesis and functionality of the cell wall of lactic acid bacteria, Antonie van Leeuwenhoek, 76, 159–184. |
| [43] | Horne, M. T., Richards, R. H., Roberts, R. J., Smith, P. C., 1977, Peracute vibriosis in juvenile turbot Scophthalmus maximus, J. Fish Biol., 11, 355–361. |
| [44] | Lupiani, B., Dopazo, C. P., Ledo, A., Fouz, B., Barja, J. L., 1989, New syndrome of mixed bacterial and viral etiology in cultured turbot Scophthalmus maximus, J. Aquat. Anim. Health, 1, 197–204. |
| [45] | Lee, H. K., Kim, H. J., Kim, I., 1991, Isolation of Vibrio species from cultured flounder (Paralichthys olivaceus) with ulcers and ascites in the southern coast of Korea during the winter season, Korean J. Microbiol., 29, 319–328. |
| [46] | Hjelm, M., Bergh, O., Riaza, A., Nielsen, J., Melchiorsen, J., Jensen, S., Duncan, H., Ahrens, P., Birkbeck, H., Gram, L., 2004, Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scophthalmus maximus) rearing units, Syst. Appl. Microbiol., 27, 360–371. |
| [47] | Chair, M., Dehasque, M., Van Poucke, S., Nelis, H., Sorgeloos, P., De Leenheer, A. P., 1994, An oral challenge for turbot with Vibrio anguillarum, Aquac. Int., 2, 270–272. |
| [48] | Grisez, L., Reyniers, J., Verdonck, L., Swings, J., Ollevier, F., 1997, Dominant intestinal microflora of sea bream and sea bass larvae, from two hatcheries, during larval development, Aquaculture, 155, 391–403. |
| [49] | H. Chart, C. B. Munn, Experimental vibriosis in the eel (Anguilla anguilla). In: Ahne W, Ed. Fish Diseases. Heidelberg: Springer, pp39–44, 1980. |
| [50] | Baudin-Laurencin, F., and Germon, E., 1987. Experimental infection of rainbow trout, Salmo gairneri R., by dipping in suspensions of Vibrio anguillarum, ways of bacterial penetration; influence of temperature and salinity, Aquaculture, 67, 203–205. |
| [51] | Kanno, T., Yasunoby, H., Okadak, N., Muroga, K., 1989, Mode of transmission of vibriosis among ayu Plecoglossus altivelis, J. Aquat. Anim. Health, 1, 2–6. |
| [52] | Olsson, J. C., Joborn, A., Westerdahl, A., Blomberg, L., Kjelleberg, S., Conway, P. L., 1996, Is the turbot, Scophthalmus maximus (L.), intestine a portal of entry for the fish pathogen Vibrio anguillarum? J. Fish Dis., 19, 225–234. |
| [53] | Alexander. C. P., Kirubakaran, C. J. W., Michael, R. D., 2010, Water soluble fraction of Tinospora cordifolia leaves enhanced the non-specific immune mechanisms and disease resistance in Oreochromis mossambicus, Fish Shellfish Immun., 29, 765–772, doi: 0.1016/j.fsi.2010.07.003. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML