-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(3): 131-134
doi:10.5923/j.als.20140403.06
In vitro Shoot Initiation and Multiplication of Jojoba Simmondsiachinensis (Link.) Schneider
Rania S. El Sanousi1, Samah M. Hassan2, Ismail A. Mohammed1
1Department of Botany and Agricultural Biotechnology, Faculty of Agriculture, University of Khartoum, Khartoum North, Postal Code 13314. Sudan
2Commissions for Biotechnology & Genetic Engineering, National Centre for Research, Khartoum, P.O. Box 2402, Sudan
Correspondence to: Ismail A. Mohammed, Department of Botany and Agricultural Biotechnology, Faculty of Agriculture, University of Khartoum, Khartoum North, Postal Code 13314. Sudan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
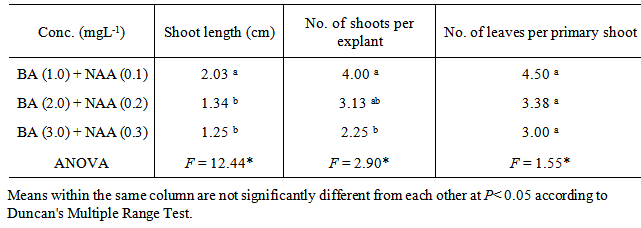
In vitro clonal propagation of a one year-old jojoba tree through axillary shoot proliferation was achieved. Nodal segments, approximately 3 cm long; each including one node with two axillary buds; were used as explants. The nodal segments were cultured on MS medium supplemented with three concentrations of BA (1.0, 2.0, or 3.0 mg L-1) and NAA (0.1, 0.2, or 0.3 mg L-1). Different growth parameters such as number of buds, length of primary shoot (cm), and number of leaves per primary shoot were recorded. The combination of 1.0 mg L-1 BA and 0.1 mg L-1 NAA exhibited the minimum time for bud sprouting (26 days), followed by the combination2.0 mg L-1 BA and 0.2 mg L-1 NAA (29 days). While the combination of 3.0 mg L-1 BA and 0.3 mg L-1 NAA exhibited the maximum time for bud sprouting (31 days). The results revealed that the combination of 1.0 mg L-1 BA and 0.1 mg L-1 NAA resulted in minimum time for bud sprouting, maximum length of shoot, high number of shoots per explant and greater number of leaves per primary shoot.
Keywords: Axillary buds, Growth regulators, Jojoba, Nodal segments
Cite this paper: Rania S. El Sanousi, Samah M. Hassan, Ismail A. Mohammed, In vitro Shoot Initiation and Multiplication of Jojoba Simmondsiachinensis (Link.) Schneider, Advances in Life Sciences, Vol. 4 No. 3, 2014, pp. 131-134. doi: 10.5923/j.als.20140403.06.
Article Outline
1. Introduction
- Jojoba (Simmondsiachinensis), gray box bush is a long-lived, evergreen, dioecious and an obligated cross-pollinated shrub of the family Simmondsiaceae native to the Sonoran desert in Northern Mexico and Southwestern USA [1]. It tolerates drought and salinity, and it has ability to thrive in marginal soils with low nutrient requirements and little care [1], [2]. Jojoba plant has a long lifespan (100–200 years). The economic value of jojoba comes from its seeds which contain about 50–60% of a light yellow, odourless wax ester commonly referred to as jojoba oil [3]. The liquid wax has been proved to be an excellent replacement of whale sperm oil [4]. It also has been used commercially in lubricants, cosmetics and pharmaceutical [5]. Moreover, jojoba oil has received more attention in recent years for sustainable biodiesel production without harmful of the environment.Jojoba can be propagated directly by seeds or either by vegetative propagation techniques. Seed propagation results in genetic heterogeneity and half of the seedlings are males; therefore, this ratio is unfavourable for high productivity of jojoba [6]. Sex can be recognized only when plants start flowering after 3 to 7 years from germination [7]. Jojoba can be propagated in vivo through air-layering, grafting and stem cuttings, but the maximum number of propagules is limited by plant size and time of year [8], [6]. Therefore, successful regeneration of plants from cells and tissue culture has immense potential for the improvement of jojoba plant. The objective of this study was to investigate the effects of different growth regulators combinations on the growth and development of cultured-jojoba nodal segments in vitro.
2. Materials and Methods
- This study was carried out in Tissue Culture Laboratory, Khartoum North, Sudan.
2.1. Plant Material and Surface Sterilization
- The explants were taken from 6 months to 1 year-old jojoba plants that were growing at Faculty of Agriculture, University of Khartoum (Lat. 15˚35 ´N, Long. 32˚31´E), Sudan. Nodal segments, approximately 3 cm long; each including one node with two axillary buds; were used as explants. After removing leaves, the cuttings were thoroughly washed in running tap water for 30 min then sterilized in 75% ethanol for 3 min followed by submerging in 15% sodium hypochlorite solution plus 0.1% Tween-20 for 30 minutes. Finally explants were rinsed in sterile distilled water three times under laminar flow.
2.2. Shoots Initiation and Proliferation
- Explants were aseptically placed on Murashige and Skoog (MS) medium [9] containing 3% (w/v) sucrose,10 mgL-1 thiamine, 100 mgL-1 myo-inositol,0.7% (w/v) agar, and different combinations of BA (N6-Benzyladenine) with auxin (α-Naphthaleneaceticacid) as listed below.A. MS + (1.0 mgL-1) BA+ (0.1 mgL-1) NAA B. MS+ (2.0mgL-1) BA+ (0.2 mgL-1) NAAC. MS+ (3.0 mgL-1) BA+ (0.3 mgL-1) NAAThe pH of the media was adjusted to 5.7 using either0.1 N NaOH or HcLbefore to adding 0.7% (w/v) agar. The culture tubes were wrapped and autoclaved at 1.06 kg cm and 121°C for 15 min. Cultures were incubated under a 16 h light (31-35 µmol photon m-2 s-1)/ 8 h dark photoperiod at 25± 2°C. Each treatment consisted of 8 explants and each treatment was repeated three times. Sub-culturing was carried out every 14 days for two and half month on the same shooting initiation and multiplication media.The data were recorded from clean cultures on the following parameters; number of buds, length of primary shoot (cm), and number of leaves per primary shoot. The mean, standard error and ANOVA analysis were calculated using SAS programme. Significant differences between means among treatments were compared using Duncan’s multiple range tests and significance was determined at P ≤ 0.5.
3. Results and Discussion
- Although the treatment of cuttings in jojoba plant with different growth regulators will increase the total number of propagules obtained from a stock plant [10], yet the maximum number of possible propagules is limited by plant size and time of year [11], [12]. Therefore, in vitro propagation could be a good solution for mass production of desirable elite genotype of plants from the selected stock plant [12] in a short possible time independent of the season. In this study, nodal segments were used asexplants for initiation and proliferation of axillary buds in S.chinensis. Several studies have been reported the usage value of nodal segments for in vitro propagation of jojoba plant [13], [14], [15], [16]. Nodal segments were cultured on solidified MS medium supplemented with three different cytokinin and auxincombinations for shoot initiation and multiplication (Table 1). After two and half months, data regarding number of buds, length of primary shoot (cm), and number of leaves were recorded and subjected to statistical analysis. The combination of 1.0 mg L-1 BA and 0.1 mg L-1 NAA exhibited the minimum time (26 days) for bud sprouting as shown in (fig.1), followed by the combination2.0 mg L-1 BA and 0.2 mg L-1 NAA (29 days). While the combination of 3.0 mg L-1 BA and 0.3 mg L-1 NAA exhibited the maximum time for bud sprouting (31 days). The combinations of cytokinin and auxin were not significantly affected the number of days to bud sprout, because the variation among the combinations is not high. On the other hand, [17] found that the combination of 0.5 mg L-1 Kinetin and 1.0 mg L-1 BA sprouted the axillary bud within a month which was almost similar in time to bud sprouts by combinations in this study.
 | Figure 1. (a) and (b) Four -week-old single node cultures on MS 1.0 mg 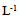 BA + 0.1 mg BA + 0.1 mg 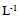 NAA showing the initiation of shoot. (c) & (d) Shoot multiplication on MS 1.0 mg NAA showing the initiation of shoot. (c) & (d) Shoot multiplication on MS 1.0 mg 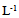 BA + 0.1 mg BA + 0.1 mg 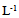 NAA NAA |
|
4. Conclusions
- The effect of BA in combination with NAA was almost similar for shoot initiation and multiplication. However the lowest combination caused early sprouting of buds that resulted in the longest primary shoot, highest number of shoots per explant and greatest number of leaves per primary shoot. Therefore, this combination would be better than other combinations for invitro propagation of elite Sudanese jojoba line.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML