-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(3): 119-122
doi:10.5923/j.als.20140403.04
The Advance of Culex quinquefasciatus (Say) into Rural Eco-vegetational Zones in Bayelsa State, Nigeria
Amawulu Ebenezer1, 2, M. Aline E. Noutcha1, Samuel N. Okiwelu1
1Entomology & Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
2Current Address: Isaac Jasper Adaka Boro College of Education, Bayelsa State, Nigeria
Correspondence to: Samuel N. Okiwelu, Entomology & Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background: Culex quinquefasciatus had always been characterized as an urban vector of Bancroftian filariasis in West Africa. However, there have been records of limited entry into rural pockets in East and West Africa. Studies were therefore undertaken to investigate the extent of its advance and behaviour in rural areas of diverse vegetation, Bayelsa State, Nigeria. Methods: Culex quinquefasciatus were collected by the pyrethrum spray catch technique from houses where occupants had slept the previous night in rural towns/villages across ecovegetational zones (Fresh water swamp forest, brackish water swamp forest, Mangrove water forest) in Bayelsa State, Nigeria, April, 2009- March, 2010. Results: A total of 2084 Culex quinquefasciatus, approximately 4-fold the number of Anopheles gambiae s.l. was collected. More were collected in the wet than dry season and the difference was significant (χ2cal. = 403.9, df=1, p<0.05). It occurred across the three eco-vegetational zones; differences were not significant. The dominant gonotrophic stage was unfed (57.1%); the other three stages were: fed (20.9%), gravid (11.1%) and half-gravid (10.9%). Indoor resting densities across the eco-yegetational zones were 24.2-47.0 mosquitoes/room/night. Spatial variation in densities was not significant. Man-biting rates were 7.6-13.2 bites/person/night. Differences in man-biting rates were significant (t=12.083, df=6, p=0.00); higher densities and biting rates were recorded in the wet season. Conclusion: The rural advance of Culex quinquefasciatus into different eco-vegetational zones is more extensive than envisaged. Based on the high densities, behavioural attributes of endophagy and endophily, it may become an important vector of rural lymphatic filariasis in West Africa.
Keywords: Culex quinquefasciatus, Rural, Advance, Behaviour, Nigeria
Cite this paper: Amawulu Ebenezer, M. Aline E. Noutcha, Samuel N. Okiwelu, The Advance of Culex quinquefasciatus (Say) into Rural Eco-vegetational Zones in Bayelsa State, Nigeria, Advances in Life Sciences, Vol. 4 No. 3, 2014, pp. 119-122. doi: 10.5923/j.als.20140403.04.
Article Outline
1. Introduction
- Wuchereria bancrofti and Brugia malayi are unique among the various mosquito- transmitted parasites; larval development can take place in several genera of mosquitoes [1]. Globally, three transmission zones are recognized where each of the three genera (Aedes, Anopheles, Culex) predominates. In West Africa, Anopheles mosquitoes are the principal vectors whereas in East Africa, transmission is mainly by Culex quinquefasciatus and other members of the Culex pipiens complex [2]. However, Cx. quinquefasciatus is considered an urban vector of the disease in West Africa, breeding in urban polluted waters [3, 4]. There have been recent reports of Cx. quinquefasciatus distribution increasing with urbanization and human activity, and many rural pockets that were relatively free of this species are now increasingly colonized [5, 6]. The available record of this rural advance in Nigeria was in the lowland rain forest [5]. Malaria and lymphatic filariasis are endemic in Bayelsa State [7]. The distribution and behaviour of Cx. quinquefasciatus were investigated during studies on Anopheles gambiae complex and malaria transmission in semi-urban/rural settings across the eco-vegetational zones in Bayelsa State.
2. Materials and Methods
2.1. Study Area
- Bayelsa state occupies an area of 21,110km2, comprising three eco vegetational zones (Mangrove Water forest, Fresh water swamp forest and Brackish water swamp forest) there are two seasons: rainy (March-October) and dry (November- February).
2.2. Methods
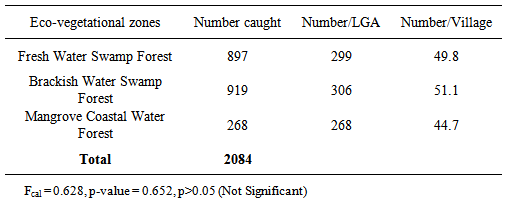
- The sampling locations were in semi-urban and rural villages located in 7 Local Government Areas (LGAs)- Nembe, Yenagoa, Sagbama, Kolokuma/Opokuma, Ogbia, Ekeremor, Southern Ijaw. The spread across eco-vegetational zones is: Mangrove coastal water forest (Nembe); Fresh water swamp forest (Yenagoa, Sagbama, Kolokuma/Opokuma); Brackish water swamp forest (Ogbia, Ekeremor, Southern Ijaw). Six towns and villages were randomly selected from each LGA for a total of 42 towns /villages (Fig 1). Permission to sample was obtained from the State Ministry of Health, through the Directors, Primary Health Care (PHC) centres in the LGAs. Pyrethroid spray catches were used in the randomly selected houses in each town/village. Verbal consent was obtained from the household heads.
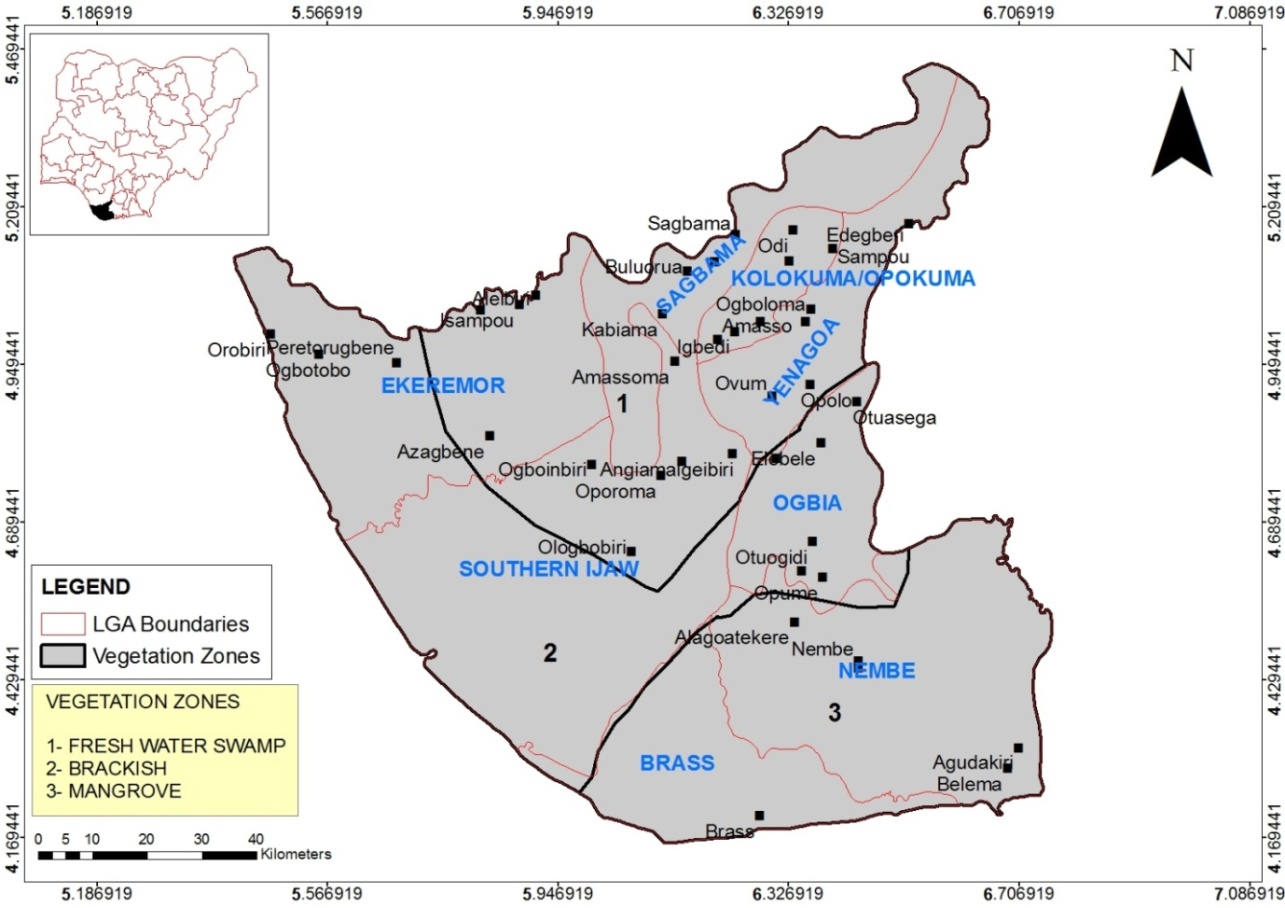 | Figure 1. Study sites in respective Local Government Area |
2.3. Statistical Analyses
- Chi square test was used to determine the significance of differences in seasonal adult abundance while the t-test was applied to differences in man-biting rates across towns and villages. ANOVA was applied to differences in adults’ abundance across eco-vegetational zones, gonotrophic stages and spatial variation in indoor resting density (ird)
3. Results
3.1. Distribution and Abundance
- A total of 2084 Cx. quinquefasciatus were caught, April, 2009- March, 2010; 519 (24.9%) and 1565 (75.1%) were collected in the dry and rainy seasons respectively. The monthly numbers were 129.75 and 195.63 in the dry and rainy seasons respectively. Seasonal differences were significant (χ2cal. = 403.9, df=1, p<0.05). Cx. quinquefasciatus occurred across the 3 eco-vegetational zones (Table 1). Differences in numbers across eco vegetational zones were not significant (Fcal. =0.628; p-value 0.652, p>0.05).
|
3.2. Gonotrophic Status
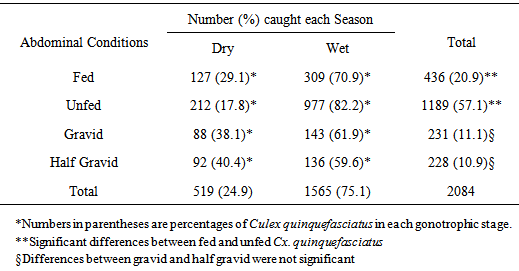
- When the numbers of Cx. quinquefasciatus in the different gonotrophic stages were pooled, 57.1% were unfed, 20.9% fed, 11.1% gravid and 10.9% half gravid (Table 2). Differences in the gonotrophic stages were significant (Fcal.=18.174, df=3, p=0.000).
|
3.3. Indoor Resting Density (ird) and Man Biting Rate (mbr)
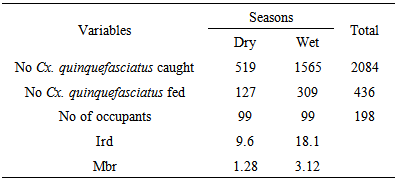
- Indoor resting densities were 24.2, 29.8, 41.1, 41.7, 46.1 and 47.0 mosquitoes/ room /night at Yenagoa, Nembe, Ekeremor, Oporoma, Ogbia, Kaiama and Sagbama respectively (Table 3). These densities were significantly higher than those of An. gambiae s.l. collected simultaneously [11]. Spatial variation in ird was not significant (Fcal. = 1.365, df = 6, p>0.05). Man biting rates varied, 7.6-13.2 bites/person/night. Highest mbr was at Ogbia while the least at Yenagoa (Table 3). Differences in man-biting rates across towns/villages were significant (t=12.083, df=6, p=0.00). Highest percent of fed mosquitoes was at Kaiama (Table 3). There were 2-fold or higher increases in irds and mbrs from the dry to the rainy season (Table 4).
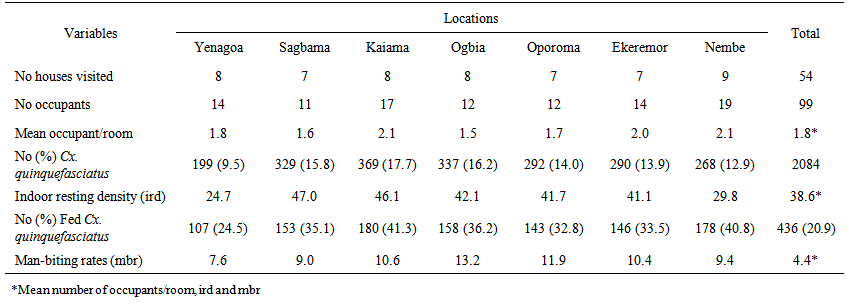 | Table 3. Indoor resting density (ird) and man-biting rate (mbr) of PSC-Culex quinquefasciatus in towns/villages September, 2009- August, 2010 |
|
4. Discussion
- Surprisingly, the numbers of Cx. quinquefasciatus were about 4-fold the numbers of Anopheles gambiae s.l. collected during the same period [10]. The invasion of rural areas by Cx. quinquefasciatus, recorded at rural pockets in Tanzania [5], in the lowland rainforest, Rivers State, Nigeria has extended further to diverse vegetation zones [6]. The invasion of rural areas by Cx. quinquefasciatus is more extensive than originally thought. Reproduction occurred throughout the year in both dry and wet seasons, although numbers were higher during the rainy season. It was therefore not surprising that indoor resting densities and man-biting rates were higher in the wet season. In semi-urban Yenagoa and rural Sagbama, Kaiama, Ogbia, Oporoma, Ekeremor, and Nembe, Cx. quinquefasciatus was both endophilic and endophagic, similar observations had been reported [3]. The feeding and resting behaviours indicate that it may be a significant vector in rural Bancroftian filariasis in West Africa. Since Cx. quinquefasciatus is dominant in some of these rural areas, it may displace An. gambiae complex from parts of its range.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML