-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(3): 114-118
doi:10.5923/j.als.20140403.03
Therapeutic Antibody Case Study: Humanized Xolair
Stephen Kugbere Agadagba
Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Correspondence to: Stephen Kugbere Agadagba , Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This review focuses on the production, mode of action, possible toxicity, current and future research of xolair as a humanised recombinant DNA-derived monoclonal anitibody. Xolair is an anti-allergy drug predominantly used as a therapy for severe persistent allergic asthma, mediated by immunoglobulin E (IgE). The drug is produced as an injectable liquid that selectively binds to circulating human IgE, thereby reducing the release of inflammatory mediators from mast cells and basophils. A possible and major toxicity of xolair is anaphylaxis owing to the small portion of mouse antibody in xolair make up. Consequently, physicians usually recommend a brief period of observation after administration of xolair injections, to rule out anaphylaxis. Xolair as a humanised monoclonal antibody is currently gaining much recognition as an anti-allergy drug therapy and is under constant clinical trials for future use in treatment of newly discovered IgE mediated allergies that are refractory to first-line drugs.
Keywords: Xolair/Omalizumab, Humanised monoclonal antibody, Immunoglobulin E, Allergies
Cite this paper: Stephen Kugbere Agadagba , Therapeutic Antibody Case Study: Humanized Xolair, Advances in Life Sciences, Vol. 4 No. 3, 2014, pp. 114-118. doi: 10.5923/j.als.20140403.03.
Article Outline
1. Introduction
- Xolair (commercial name) or Omalizumab/rhuMAb-E25 (scientific name) is a humanized therapeutic monoclonal antibody product commonly used for treatment of asthma aggravated by allergens. Xolair is normally used untagged and it specifically targets the FcεRI receptor binding portion on immunoglobulin E (IgE). Administration of xolair is by a well-trained physician via subcutaneous (under the skin) injection. Age restriction for xolair is for patients age 12 and older [1] who have a poor response to inhaled anti-asthma steroids, and have an abnormal allergen test [2].Xolair was granted approval for use in the United States, by Food and Drug Administration (FDA) in June, 2003 [1]. European approval was later secured in October, 2005, were the product was to be administered as a supplementary therapy for moderate to severe allergic asthma in adolescents and adults [3], [4]. Currently, xolair is licensed for production by Genentech and Novartis Corporations.
2. Method of Production
2.1. Preparation of Mice Hybridomas
- The first step in the production of xolair involved the creation of anti-IgE monoclonal antibodies from mice (MAE-11), which binds the FcεRI receptor binding portion on human IgE.The mice were injected with human IgE, after which the B-cells (which were then reactive to human IgE) were extracted from the spleen, and hybridized with myeloma cells to form the hybridomas (which contained the required antibody MAE-11). Next, the hybridoma cells were then cultured in a medium containing Hypoxanthine Aminopterin Thymidine (HAT). After growth, propagation and selection, a single cell line of the hybridomas was used to generate large amounts of the antibody of interest [5]. This hybridoma cell line was then cloned, and stored up by freezing, for the next stage of production.
2.2. Identification of the Heavy and Light Chain Aminoacid Sequences in MAE-11
- At this stage, reverse transcriptase was used to produce complementary DNA (cDNA) from RNA isolated from selected hybridoma cells. This process involved successfully using gene specific primers for the V-regions of mouse anti-IgE monoclonal antibody (MAE-11), in a reverse transcription polymerase chain reaction (RT-PCR) [6]. This resulted in cloning of (MAE-11) genes that encode for the three CDRs in each heavy chain variable (VH) and light chain variable (VL) regions. During the process, a large portion of the MAE-11 was also cleaved, leaving only the complementarity determining regions (CDRs) which are involved in antigen binding. Identification of the aminoacid sequences in the CDRs was thus achieved by comparism with standard sequences in the CDRs of several other immunoglobulins.
2.3. Construction of Humanised Anti-IgE Expression Vector (pdhl2)
- The first step of the stage was to construct the two cassettes for the heavy (VH) and light chain variable region (VL) genes of human IgG1. In other to do this, two other vectors, VKpBR and VHpBS were assembled. VKpBR was used to construct the (VL) expression cassettes. This vector also had two restriction sites that enabled human IgG1 VL genes to be conveniently inserted. VHpBs was used to construct the (VH) expression cassettes. Similarly, this vector also had restriction sites for the convenient insertion of human IgG1 VH genes. The two cassettes, together with sequence units in each vector, were then assembled and subcloned, resulting in the expression and secretion of the cassettes in the final plasmid vector PdHL2 [6].The cloned genes from MAE-11 where then ligated into the constructed pdHL2 plasmid vector cassettes containing genes encoding for the VL and VH (variable region frame work) human IgG1 chains arranged in tandem. In addition to these components, the plasmid also had a region that encoded for a mutant mouse dihydrofolate reductase, used as a drug-resistance marker for selection of transfectants and for increasing antibody production by gene amplification [6].
2.4. Creation of Xolair Producing Transfectomas
- Subsequently, the fully constructed vectors (pdHL2) were transfected into a Chinese Hamster Ovary cell lines in other to express the recombinant humanized anti-IgE monoclonal antibody (omalizumab/rhuMabE-25). After the cells were cultured in a medium containing gentamicin [7] and grown for about two weeks, xolair was secreted into the medium. The xolair in the supernatant from the colonies was analysed by Enzyme Linked Immunosorbent Assay (ELISA), to check antigen (Ag) binding specificities. Positive colonies that showed high Ag-binding specificities similar to the parent MAE-11 were then selected by plating with methotrexate. Such desired colonies were used to scale up production of xolair by transferring the cells to large industrial production tanks [1] for further processing.
2.5. Purification of Xolair
- Purification was achieved by chromatographic techniques including: immobilized protein A column chromatography, cation exchange chromatography (on SP sepharose) and anion exchange chromatography (on Q sepharose) [8]. These three techniques were done sequentially. After anion exchange chromatography, the resulting eluate was concentrated by ultra-filtration and diafiltration processes [8]. The final product obtained after this, was high purity xolair (consisting of 95% human IgG framework and 5% mouse MAE-11 CDR sequence) which was channelled for storage in large vats.
2.6. Binding Characteristics
- Xolair monoclonal antibodies were analysed in various binding assays. Such assays included ELISA and Florescence Activated Cell Sorter. Invitro studies with several cell types such as cells from cynomolgus monkeys, showed that xolair competes with FcεRI receptors for binding on IgE (EMA, 2005). In these studies, the dissociation constant (Kd) of xolair for IgE binding usually varied between (0.02 × 10-9 mol/L) and (7.7 × 10-9 mol/L), depending on the type of assay used [8].
3. Mode of Action
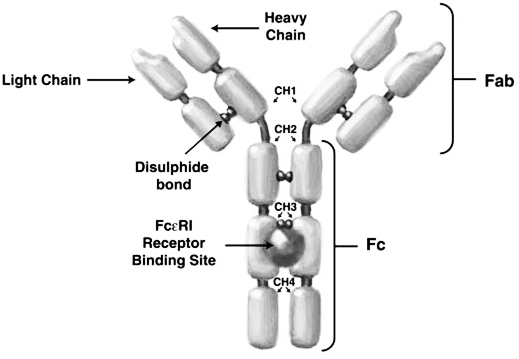
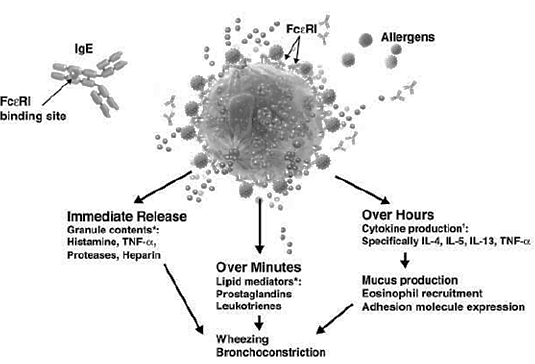
- Immunoglobulin E (IgE) (figure 1) has been implicated in mediating many of the inflammatory responses which entail the symptoms of asthma and many other allergic respiratory disorders [5] In the process, when the patient is initially exposed to allergens, IgE is released and binds to FcεRI receptors on mast and basophil cells, to form IgE-FcεRI complexes, which initially causes no allergic response (figure 2). However, subsequent exposure causes the allergens to bind to the pre-formed IgE-FcεRI complexes, thus triggering the release of inflammatory molecules like histamines, prostaglandins, leukotrienes, and so on from cells (figure 2). It is the release of these mediators that leads to inflammation, oedema, bronchospasm, and excess mucous production which worsen asthma [9]. Xolair therefore targets the FcεRI binding region on human IgE and stops IgE from binding to FcεRI receptors on mast and basophil cells, thus inhibiting formation of cross-links or complexes between IgE and such cells. It has been suggested that xolair affects mast cell stability by blocking calcium ion (Ca2+) channels that have functional roles in degranulation of mast cells [5]. Specifically, xolair blocks the IgE-regulated Ca2+ channels. In the absence of intracellular Ca2+, histamine vesicles are unable to bind the cell membrane and degranulate.
3.1. Therapeutic Roles of Xolair in Non-allergic Conditions
- A significant number of current research works have published data from clinical trials of xolair which suggest that the drug may have positive effects in patients with non-allergic asthma [18]. However, this is quite contrary to the general understanding of xolair’s mode of action as discussed above. Besides, among the diseases in which xolair has been studied for efficacy and safety, some are not allergic conditions, because hypersensitivity responses towards external antigens are not involved. Typical examples of this are observed in autoimmune diseases such as some cases of chronic idiopathic urticarial [19], [20] and virtually all cases of bullous pemphigoid [21]. For the remaining cases of chronic idiopathic urticaria and those of the different subtypes of physical urticaria, the internal abnormalities leading to the disease manifestation are yet to be identified. Despite these developments, it is clear that many of these conditions still involve inflammatory reactions in the skin and the activation of mast cells. Therefore, xolair may have beneficial roles in non-allergic conditions because as described above it functions as a mast cell-stabilizing agent, thereby rendering these inflammatory mast cells to be less active.
4. Toxicity of Xolair
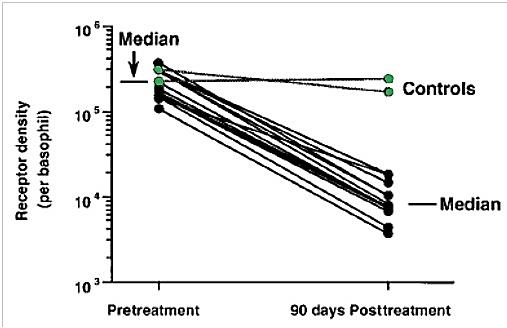
- Although several possible toxicities of xolair have been observed in the minority (< 0.5%) of patients tested during clinical trials, however the major contraindication of xolair is anaphylaxis as described below.Anaphylactic tendency: Xolair like most other humanized therapeutic antibodies may cause serious anaphylactic reactions owing to the small portion (5%) of mouse antibody CDR framework in xolair molecule, which could trigger an immune response when administered in humans. Serious anaphylactic and anaphylactoid reactions sometimes leading to death, have been reported [7] within few hours of administering xolair injections. For this reason, physicians usually recommend a period of observation for patients, after xolair injections. It has been reported that IgE could have roles in the immune system's recognition cellular carcinomas [15]. In this regard, a general reduction of cellular IgE levels and immunity by xolair may have unforeseen risks. For instance, a study carried out in 2003 from early phase I to phase III clinical trials of xolair, showed a numeric imbalance in malignancies arising in patients receiving xolair injections (0.5%) compared with control recipients (0.2%) [16]. In order to confirm this imbalance, a more recent study was carried out on the basis of pooled analysis using broader data from 67 phase I to IV clinical trials. The pre-specified primary analysis assessed the incidence of primary malignancy in 32 randomized, double-blind, placebo-controlled (RDBPC) trials. In this analysis, there were 11,459 unique patients in all clinical trials (7,789 received xolair). The primary analysis identified malignancies in 25 patients (RDBPC trials): 14 in 4,254 xolair treated patients and 11 in 3,178 placebo-treated patients. Incidence rates per 1,000 patient-years of observation time for xolair and placebo-treated patients were 4.14 (95% CI, 2.26-6.94) and 4.45 (95% CI, 2.22-7.94), respectively; the corresponding rate ratio was 0.93 (95% CI, 0.39-2.27). Primary malignancies were of varying histologic type and occurred in a number of different organ systems; no cluster of histologies was identified. Finally, the study concluded that in the pooled analysis, no association was observed between xolair treatment and risk of malignancy in RDBPC trials; the rate ratio was not significant. The data suggest that a causal relationship between xolair therapy and malignancy is very negligible.
5. Conclusions
- The development of xolair as a humanized therapeutic antibody is an advancement over the chimeric therapeutic antibodies. Recently, xolair has been approved by the European Commission and the FDA as a second-line therapy for the treatment of chronic spontaneous urticarial (hives) that is resistant to oral antihistamine drugs [14]. Hives commonly occur due to the effect of histamines on H1-receptors found in the endothelial cells lining blood vessels. Histamines lead to cell separation which in turn causes the leakage of tissue fluid that culminates in the formation of wheals in hives. Histamines affect sensory nerve cells, causing neurogenic flare (red skin) and pruritus (itch), which are also characteristic symptoms of hives. As mentioned previously, xolair uses a similar anti-allergen mechanism for the treatment of hives. Xolair prevents histamines from binding to high affinity receptors (FcεRI) on the surface of mast cells and basophils, thus reducing receptor expression, inhibiting the release of inflammatory mediators and ameliorating the symptoms of hives. Although, a very recent study [22] has identified MEDI4212; a novel anti-IgE monoclonal antibody that works via the same mechanism as xolair for the treatment of severe uncontrolled asthma with high total IgE levels or body mass, future studies are still looking into xolair as a second-line therapy for other conditions with high levels of autoimmuno-IgE antibodies such as atopic dermatitis and bullous pemphigoid. In this regard, clinical trials are however warranted.
ACKNOWLEDGEMENTS
- Special thanks and appreciation go to my beautiful fiancée, Precious Onuwaje for her love, patience and support. Love you darling.
Conflict of Interest
- The author declares no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML