-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(3): 101-105
doi:10.5923/j.als.20140403.01
Biological Control of Water Snails by Dragonfly Nymphs
K. P. Singh
Centre for Wildlife Forensic and Health, Nanaji Deshmukh Veterinary Science University, South Civil Lines, Jabalpur, 482001, India
Correspondence to: K. P. Singh , Centre for Wildlife Forensic and Health, Nanaji Deshmukh Veterinary Science University, South Civil Lines, Jabalpur, 482001, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
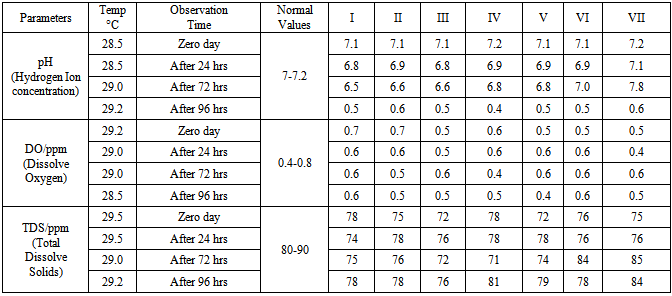
The predatory potential of dragonfly nymphs was studied on water snails (Indoplanorbis exustus and Lymnaea luteola) and their larval stages under laboratory conditions. The water snails (snail’s cocoons, young snails and adult snails etc.) and dragonfly nymphs were collected from stagnant water resources of protected areas and brought to laboratory, maintained with aquatic weeds and mulberry leaves in the artificial cement tank. The collected water bodies were used in the requisite quantity in glass containers of 6 litre water capacity each with snails, aquatic weeds and dragonfly nymphs for trial groups while the container of control group was kept without dragonfly nymphs. Observation recorded from 0, 3, 6, 12, 24, 48, 72 and 96 hrs of nymphs entry while feeding activity of nymphs was judged by counting the remaining numbers of snails and their larval stages in the different containers. The pond water quality analysis was envisaged, average dissolve oxygen (DO- 0.4—0.7 ppm), hydrogen ion concentration (pH-6.7-8.7) and total dissolve solids (TDS-71-84 ppm) during the entire period of experiment. Remarkable reduction (82-97 %) in the cocoons and young snail population were noticed whereas 18-40% mortality have also encountered in the snails of control group. The predacious nature and potentials of the dragonfly nymphs may be promoted as one of the appropriate biological control agent to overcome the snail borne parasitic diseases.
Keywords: Biological Control, Water Snails, Nymphs, Dragonfly
Cite this paper: K. P. Singh , Biological Control of Water Snails by Dragonfly Nymphs, Advances in Life Sciences, Vol. 4 No. 3, 2014, pp. 101-105. doi: 10.5923/j.als.20140403.01.
Article Outline
1. Introduction
- The dragonflies (Palaeophlebia) are useful predacious insect and their nymphs grow in the fresh water resources like rainy season ditches, river pools, ponds and marshy lands etc. Similarly the water snails (Indoplanorbis exustus and Lymnaea luteola) are also habitat to the stagnant water resources [1, 2]. The fluke eggs usually passed in the faeces of domestic as well as wild herbivores and under suitable conditions of moisture and warmth larva called miracidia hatches and infect the water snails that act as intermediate host [15]. Expansions of parasitic diseases are worldwide owing to the human rehabilitation and indiscriminate uses of natural resources. Fluke borne diseases are creating an alarming situation to sustenance of domestic and wild ruminants with the loss of agility due to apoptosis in the infected host cells that decrease the immunocompetency resulting untimely demises [5]. Frequent breeding of snails around the human community and their livestock occurs due to lift irrigation, pool of water and conventional water logging system in the rural areas. Thus it became major problem to the animal industry growth as worldwide 300 million bovines found annually exposed with the fluke parasites causing economic losses amounting more than 3.0 Billion US$ [7]. Inadequate uses of chemotherapeutics in practices with the reliance on chemical control have commenced harmful circumstances to the ecosystem with residual toxicity and resistance against the parasitic infections in animals [4, 8]. Withstanding the facts of drug resistance, the biological control measures are reasonably appropriate as they are eco-friendly and beneficial for sustenance of aquatic flora and fauna. There are several biological control agents found in the fresh water resources like larvivorous fishes, water bugs, Coleopteran aquatic larvae, Crayfish, Cyclopes and Echinostome snails etc., those naturally helps in controlling the population of snails though they have their own barriers and capacity [9]. However, the sustainable alternative needs to have distinct advantages and ability to kill the target species with easy applications, less expensive and non-pathogenic to biotic community [6]. Such qualities have together with the dragonflies and their larval stages as they are entomophagus in nature and frequently feed upon snails with longevity to spend their three- forth life span (1-3 years) in the aquatic habitat [10]. Michelson [11], Somasunderao [14], Bali et al. [3] have used dragonfly nymphs as biological control agent to overcome the schistosome bearing snails while Singh et al. [13] also used for control of malaria vector.In the present study, an attempt was made to assess the predatory potentials of dragonfly nymphs as biological control of water snails.
2. Materials and Methods
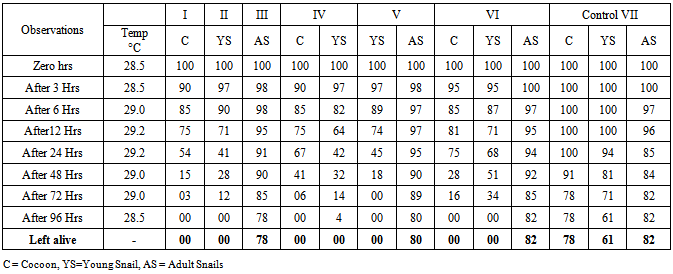
- A series of laboratory based experimental trials on dragonfly nymphs were conducted since 2010, aiming with daily predation rate, predatory preferences on snail cocoons, small and large size snails, selective predation on different species of snail’s and their dietary requirements etc.The water snails (Indoplanorbis exustus and Lymnaea luteola) with their larval stages and dragonfly nymphs were collected from stagnant water resources of protected areas and maintained into artificially made cement tank with aquatic weeds. Further they were kept in different classified containers with aquatic weeds, lotus and mulberry leaves as the snails are frequently feed upon the mulberry leaves and shelter their cocoons under the surface of leaves of Lotus (Nilumba nucifera) and water chestnuts (Trapa natans). After repeated outcomes and encouraging observations, the laboratory experiment was scheduled in the glass containers (12”x12”x 6”) with 5-liters of stagnant water. The predatory potential of the dragonflies’ nymphs were assessed on the basis of snails feeding status with their larval stages in the each container by dragonfly nymphs. The experiment was designed with seven groups: I –Cocoons (100), II- Young snails (100), III- Adult snails (100), IV-Cocoons (100) and Young snails (100), V-Young snails (100) and Adult snails (100);VI- Cocoons (100), Young snails(100) and Adult snails (100) and VII- Cocoons (100), Young snails(100) and Adult snails (100) without nymphs of dragonfly (Control). Five nymphs of dragonfly were released in the containers of 1-6 groups and the feeding behaviour and their activities were recorded between 0-96 hrs (Table 1) while the water quality analysis (Table 2) was also performed at 0, 24, 72 and after a week intervals for Hydrogen ion concentration of the water (pH), dissolve oxygen (DO) and total dissolve solids (TDS) by commercially available water quality analysis kits (Aqua-Check Pvt. Ltd. India). However, for snail’s feed, the fresh mulberry leaves were used on alternate days and the faecal material of nymphs or dead snail’s shells were routinely discarded. For appropriate counting of dead and live snails, the method of Agrawal [1] was used as dead snails sink frequently in the jar water.
|
|
3. Results and Discussion
- The dragonfly nymphs have natural predation upon snails. They frequently feed on cocoons as well as young snails of L. luteola and I. exustus (82-97%) in I-VI groups while unable to prey on adult snails (Table 1 Fig 1). It was also noticed that nymphs are mostly prefers bottom surface where only adult snails resides and due to their big size, these nymphs are found to be incapable to neutralize the adult snails (Fig 1b). The cocoons of the snails reside under the surface of the lotus leaves and usually remain safe (Fig 1a). Survival of the larval stages of the snails in the natural water resources depends upon the ample quantity of aquatic weeds and leaves to escape from predatory insects [2] whereas in the present experiment, glass containers (12”x12”x 6”) with 5-liters of stagnant water were found to be inadequate, hence the nymphs could easily find and predate upon the larval stages of the snails. Bali et al. [3] and Somasunderao [14] have also tried the aquatic insect in the laboratory conditions for controlling the snail borne diseases of live stock with unidentified species of the insects.
 | Figure 1. A: Snails and their Cocoon on Lotus leaf B: Dragonfly Nymphs defending with adult snails C: Nymph feeding on young snails D: Nymphs fighting each other |
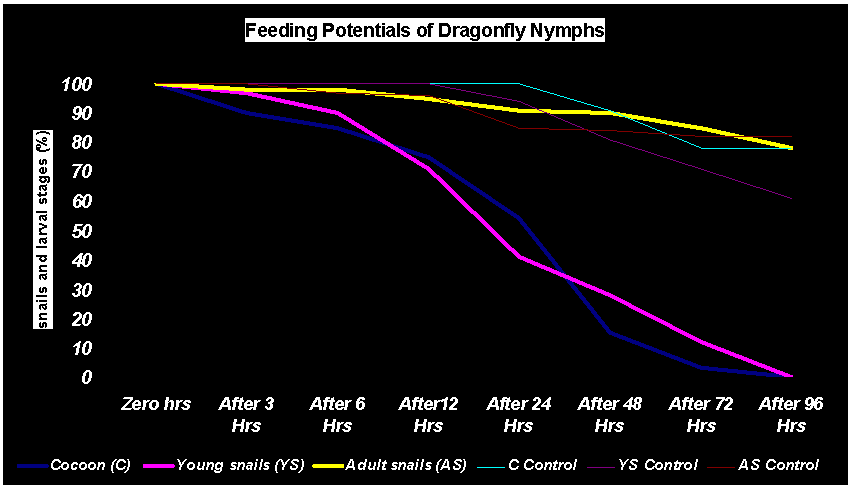 | Graph. Feeding Potentials of Dragonfly Nymphs |
4. Conclusions
- Fluke borne diseases are major threats to the livestock industry as well as wild ruminants though biological control agents have capacity to control them. The assessment of the present study is suggested for the initiation of breeding programmes for the development of huge number of dragonfly nymphs and their introduction in different ponds, ditches and marshy lands that may be succeeded to check the population of water snails.
ACKNOWLEDGEMENTS
- Author is highly obliged to Dr. M. C. Agrawal former National Fellow of ICAR and Dr. A.B. Shrivastav, Director, CWFH for their valuable suggestions. The financial support of the Centre for Wildlife Forensic and Health, Nanaji Deshmukh Veterinary Science University, Jabalpur and ICAR- NATP Mission mode project on” Immunodiagnostics of Parasitic diseases of animals are gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML