-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2014; 4(2): 52-58
doi:10.5923/j.als.20140402.03
Efficacy of a Growth Promoter Concentrate on Biochemical Parameters and Immune Response of Commercial Broilers
Hedayati M.1, M. Manafi1, M. Yari1, P. Vafaei2
1Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
2Tehran Dane Feed Manufacturing Company Ltd., Tehran, Iran
Correspondence to: Hedayati M., Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
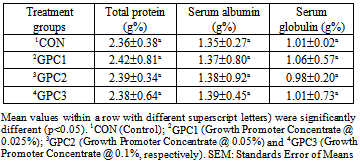
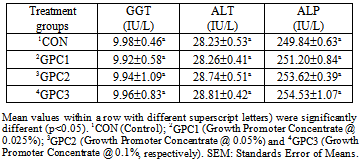
Two hundred and Forty day-old chicks were randomly distributed in a completely randomized experimental design with four treatments and three replications of twenty chicks each. Diets prepared without additive as Control (CON) (group1); 0.025% Growth Promoter Concentrate (GPC1) (group2); 0.05% Growth Promoter Concentrate (GPC2) (group3) and 0.1% Growth Promoter Concentrate (GPC3) (group4). Growth promoter concentrate contains MOS, EOs, Vitamins and essential components. Results showed that significant improvement for all antibody titers again Newcastle Disease (ND), Infectious Bursal Disease (IBD) and Avian Influenza (AI). The results of total protein, serum albumin and serum globulin showed no significant difference among the dietary treatments for these parameters. Activities of serum gamma glutamyl transferase (GGT), alanine amino transferase (ALT) and Alkaline phosphatase (ALP) also remained non-significant. It can be concluded that dietary growth promoter concentrate could have a clear positive effect on immune response but could not improve the and serum biochemical parameters; however, there was a slight positive effect on 0.1 % level of inclusion in the diet on serum biochemical parameters.
Keywords: Growth promoter, MOS, EO, Vitamin, ALT, GGT, Immunity, Broilers
Cite this paper: Hedayati M., M. Manafi, M. Yari, P. Vafaei, Efficacy of a Growth Promoter Concentrate on Biochemical Parameters and Immune Response of Commercial Broilers, Advances in Life Sciences, Vol. 4 No. 2, 2014, pp. 52-58. doi: 10.5923/j.als.20140402.03.
Article Outline
1. Introduction
- A great deal of research has been carried out to investigate the effect of antibiotic growth promoters in promoting performance parameters of broilers. Numerous studies have been conducted on antibiotic growth promoters such as Avilamycin, Virginiamycin, Lincomycin, Flavophosphol - ipol, and Bacitracin (Bedford, 2000; Elwinger et al. 1998; Salminen et al. 1998). In general, the investigations have shown different reasons concerning the effect of antibiotic growth promoters. The reduction of turnover rate of enterocytes that results in reduction of body’s energy, the reduction of immunology stress due to reduction of intestinal microflora, the competitive repression of microfloras of intestinal pathogen and promotion in absorption of nutritive foostuffs, the growth of efficient energy for production (by means of promoting AME in foodstuffs and reducing the essential energy for keeping and permanent endurance), the improvement of growth factors, the production of pathogen germs which have resistance against antibiotics whenever they are used in long-term periods, the prevention of colonizing efficient bacteria of intestine like lactobacillus and the reduction of non-specific immunity of mucous (Chen et al. 2005; Elwinger et al. 1998; Hofacre et al. 2003; Lemieux et al. 2003; Roberfroid, 1998; Salminen et al. 1998). Probiotics have recently come into the market of poultry and are a compound of live microorganisms which promote natural intestinal microflora and have a beneficial effect on broiler performance and immunomodulation (Lutful Kabir, 2009). The findings of various studies have shown that the effect of probiotics can be mentioned as: turnover of efficient microflora in digestive system (Bello et al. 2001; Lemieux et al. 2003; Vegad, 2004); changes in bacteria metabolisms (Vegad, 2004; Zoppi, 1998); neutralization of entertoxins (Vegad, 2004) and stimulation of immune system (Savage et al. 1996; Vegad, 2004). Mannan oligosaccharides (MOS) is one of the most important productions of this group. There are several studies on the effect of this substance on the immune system of poultry. In the case of prebiotics, Bailey et al. (1991) conducted a study on the effect of Fructo oligosaccharide on turnover of Salmonella in intestine mucous and mucous immune of intestine. Their findings indicated that these compounds were effective in prohibiting turnover of deleterious bacteria like Salmonella (Bailey et al. 1991). Since MOS is one of the natural products of growth stimulating from the group of prebiotics, it has no medical leftover on poultry meat. Also, with the consumption of poultry’s meat by consumers no resistance on MOS of other antibiotics is produced in individuals. Since June 1999 in Europe the consumption of most antibiotic growth promoters in poultry has been forbidden due to antibiotic leftover on meat and also producing medicinal resistance on poultry and humans. It seems that using natural compounds such as MOS that has a high efficiency can be used as one of the best alternatives for antibiotic growth promoters (Bedford, 2000; Roberfoid, 2000; Vegad, 2004; Zoppi, 1998). Synbiotic (probiotic and prebiotic) have been determined to be antimicrobial, anti carcinogenic, antiallergenic and a stimulating factor of immunity system. They also are reasons for absorption of minerals and prevention of diarrhea and optimization of nutrients’ digestion, however, synbiotics mechanism of act is generally unknown (Salminen et al. 1998). These compounds improve and increase immunity level and production factors of broiler chickens. Using these substances in poultries’ diet provide consumers with healthy meat without drug residues (Bedford, 2000). Plants are the oldest friends of mankind. Herbs and spices have always been helpful to cure diseases. In modern animal feeding, they are forgotten because of use of antimicrobial growth promoters (AGP). But due to the prohibition of most of AGP, plant extracts have gained interest in animal feed strategies (Charis, 2000). Many plants also produce secondary metabolites such as phenolic compounds, essential oils and sarasaponins (Chesson et al., 1982; Wallace et al., 1994; Kamel, 2001). There is evidence of herbs having been used in the treatment of diseases and for revitalizing body system in almost all ancient civilizations, the Egyptian, the Chinese and even Greek and roman civilizations (Aftab and Sial, 1999). Kar et al. (2004) have reported that several plant products are claimed and proved to possess analgesic and antipyretic properties. Majority of herbal plants are safe and economical. Generally, plant extracts have no problem of drug resistance. Herbs normally used are picorhiza, garlic, cloves, slippery elm, neem fruit and leaves, sophora flavescens, nutmeg, cinnamon, ginger, peppermint, sage, thyme, mustard and fenugreek. These plants are used as digestive stimulants, antidiarrhoic, antiseptic, antiinflammatory, antiparasitic and appetite stimulants in human beings as well as animals. Earlier studies indicate that many plant extracts have antimicrobial activity. According to Almas, (1999), the extracts of Azadirachta indica (neem plant) chewing sticks are effective against Streptococcus mutans and Streptococcus faecalis. Hayat et al. (2004) studied the in vitro antimicrobial activity of Zizyphus vulgaris root extract against both gram positive and gram negative organisms using Staphylococcus aureus and Escherichia coli, respectively. Three different concentrations of the ethanolic extract of the roots were used and the activity compared with the standard antibiotics. Flavonoids and phenoic acids are widely present in higher plants. These compounds are effective against the deleterious effect of reactive oxygen species. According to Middleton and Kandaswami (1993), some compounds found in Ocimum plant have been reported to possess strong antioxidant activity. Cinnamon has antioxidant characteristics (Middleton and Kandaswami, 1993). Cinnamon extracts show antioxidant activity which is comparable to synthetic antioxidants, beta hydroxy toulene. Previous literature shows that use of herbs in animal feed improved the weight gain of animals. These can be used simultaneously for treating parasitic diseases as well as increasing the weight gain and act as growth promoters. Hayat et al. (1996) studied comparative prophylactic effects of indigenous preparations of bakin (Melia azadarach) and kerala (Momordica charntia) in comparison with the salinomycin against coccidiosis in broiler chicks. The results revealed higher (P<0.05) weight gain in the birds using salinomycin and those of uninfected untreated groups. The performance index clearly depicted its efficacy at these dose levels. The efficacy was found to be higher in higher dose levels. The reaction of free radicals with polyunsaturated fatty acids (PUFAs) initiates a chain- reaction process known as lipid peroxidation. When these biochemical events occur in living systems, lipid peroxidation changes the structure of amino acids and enzymatic activities and cause damage to DNA and the structures within cell membranes (Lima and Abdalla, 2001). In foods, lipid peroxidation causes rancid flavours, changes in nutritional value and formation of toxic products, mainly aldehydes (Guedes, 2006). According to origin, antioxidants can be classified as synthetic or natural. Synthetic AOX have been widely used as food preservatives, because of their effectiveness and relatively low cost. The most used antioxidants are those derived from phenolic structures, like butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tert-butylhydroxiquinone (TBHQ) and dodecyl, propyl and octyl gallate. All of them have an admissible daily ingest (ADI). In contrast to the others, its consumption by humans is not allowed, but it is only used in animal diets such as in the preservation of aviary foods (Bailey et al., 1996). On the other hand, natural AOX are generally molecules present in plant parts (e.g. leaves, bark, seeds and/or fruits). Among the most important natural AOX are the tocopherols (or vitamin E, liposoluble) and ascorbic acid (vitamin C, hydrosoluble). While the former represents an essential nutrient (it must be consumed in the diet), the latter is biosynthesized by poultry (Pardue and Thaxton, 1986). Other natural molecules with antioxidant characteristics are carotenes (i.e. β-carotene, lycopene, luthein, asta-, zea- and cantha-xanthin), flavonoids (i.e. catechins, epigallocatechins, quercetin, rutin and morin among others), and non-flavonic phenols (i.e. rosmanol and rosmaridiphenol; boldine and its analogous). Surai et al. (1998) observed the effect of a range of supplementation with vitamin A to the laying hen on the concentration of vitamin E in the maternal liver, the egg yolk and the embryonic liver. The concentration of vitamin E in the maternal liver was markedly reduced by high dietary contents of vitamin A. In general, higher levels of vitamin A in the diet significantly reduced concentrations of vitamin E in the egg yolk and in the liver of chickens and the embryo. The susceptibility of the embryonic/neonatal liver to lipid peroxidation was significantly increased as a result of high provisions of maternal vitamin A. The authors concluded that excessive supplementation with vitamin A in laying hen diets results in an adverse effect on vitamin E in the embryonic/neonatal liver that can compromise the antioxidant status of the progeny. Grobas et al. (2002) also observed negative effect of vitamin A supplementation in the diet of hens on the concentration of alpha-tocopherol in egg yolk. This was attributed to competition between these vitamins for absorption. Vitamin E is included in animal feed to improve performance, strengthen immunological status and increase the vitamin E content of animal meat. In this respect vitamin E is used in poultry feed on the basis that vitamin E synthesis is impaired during heat stress. It was suggested that heat stress increases lipid peroxidation in poultry (Naziroglu et al., 2000), although it has been reported that vitamin E protects the liver from lipid peroxidation and cell membrane damage (Sahin and Kucuk, 2001). Vitamin E is a major chain-breaking antioxidant and an important lipid component of biological membranes (Sahin and Kucuk, 2001). It is mainly found in the hydrocarbon part of the membrane lipid bi-layer towards the membrane interface and in close proximity to oxidase enzymes, which initiate the production of free radicals. Vitamin E therefore protects cells and tissues from oxidative damage induced by free radicals (Sahin and Kucuk, 2001). The aim of the present study was to investigate the effects of different dosage of a Growth Promoter Concentrate (containing MOS, Antioxidants, plant bioactive compounds and Vitamins) as an alternative to AGP on immune response and blood biochemical parameters of broiler chickens. The efficacy of different dosage of this concentrate was also investigated in this trial.
2. Material and Methods
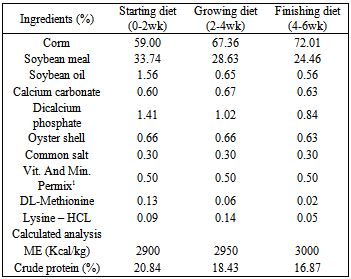
- The present study was carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with an objective of assessing the biochemical parameters and immune response commercial broilers fed with Growth Promoter Concentrate. Experimental design, Housing, Management and Test DietA total number of 240 day-old unsexed Ross 308 broiler chicks were wing banded, weighed and distributed in a completely randomized experimental design with four treatments and three replications of twenty chicks each. Each replicate group of chicks housed in an independent pen, conventional sided deep litter house. Chicks in all the replicates were reared up to six week of age under uniform standard conditions throughout the study. Brooding was done till three weeks of age using incandescent bulbs. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4 the lighting schedule was 24 h light. At days 5-49 the dark time was increased to 1 h. Basal diet was formulated and compounded to meet the nutrient requirements of commercial broilers during the starter (0-2 wks), grower (2-4 wks) and finisher (4-6 wks) feed. The composition of experimental diets is shown in Table 1. Diets prepared without additive as Control (CON) (group1); 0.025% Growth Promoter Concentrate (GPC1) (group2); 0.05% Growth Promoter Concentrate (GPC2) (group 3) and 0.1% Growth Promoter Concentrate (GPC3) (group4). ). The natural Growth Promoter Concentrate used in this study was Provital (containing essential oils, vitamins, antioxidants and MOS from natural sources) provided by a commercial company (Tehran Dane Limited, Tehran, Iran).
|
3. Vaccination Schedule
- The local office of Iranian Veterinary Organization have suggested the required local vaccination and modulated by the veterinarian of Malayer University, as below:Vaccination against Newcastle Disease (ND) virus happened three times: first spray at the commencement of experiment, second on the 12th day as B1 (CEVA SANTE ANIMALE, Libourne, France) in drinking water and booster of them on 20th day as clone-30 (HIPRAVIAR® CLON, Amer, Spain) in drinking water. Vaccination against Bronchitis virus happened in two times as the following: first spray at commencement of the experiment and the booster in drinking water on the 10th day, both as H-120 (CEVA SANTE ANIMALE, Libourne, France). Vaccination against Infection Bursal Disease (IBD) virus happened in two times: first on day 15 and the second on the 24th day, both as Gambo-l (CEVA SANTE ANIMALE, Libourne, France) in drinking water. The sera were applied to HI test in 28 the day, to determine Ab to NDV. In titers lower that 5, the booster B1 (CEVA SANTE ANIMALE, Libourne, France) was administrated in drinking water for broilers.
4. Studied Parameters
- Immunity parametersAt the end of the trials, upon obtaining the permission of Ethical Committee of the University, six birds from each replicate were sacrificed by cutting the jugular vein and blood samples were individually collected in 10-mL heparinized tubes and stored on ice for hematology analysis. Serum was separated after 8 to 10 hours as per the standard procedures (Calnek et al. 1992) and was stored at –20ºC for subsequent analysis. The individual serum samples were analyzed for antibody titers against Newcastle disease (ND), Infectious Bursal Disease (IBD) and Avian Influenza (AI) by ELISA technique and using an automatic analyzer (Boehringer Mannhein Hitachi 704 automatic analyzer, Japan). Treatment-wise means of titers were computed. Biochemical parametersThe collected blood samples were analyzed for total proteins, serum albumin, uric acid and the activities of gamma glutamyl transferase (GGT) and alanine amino transferase (ALT) using automatic analyzer (Boehringer Mannheim Hitachi 704 automatic analyzer, Japan). The methodology and the set of reagents used in respect of each parameter were as per the recommendations of the manufacturer of the analyzer system. Data are presented as means of each treatment.Statistical analysis The experimental data were analyzed statistically by using the General Linear Model procedure of the Statistical Analysis System (SAS®) software (SAS Institute, USA, 2000). Overall data were analyzed using one way ANOVA test. Duncan multiple range test at 0.05 probability level was employed for comparison of the means (Duncan, 1955).
5. Results and Discussion
- The effects of acidifier on the immune response: The results of antibody titers against Newcastle Disease, Infectious Bursal Disease and Avian Influenza are showed in Table 2. A significant (p<0.05) difference among the dietary treatments for all antibody titers has been found. The antibody titers varied from 5.00 to 5.98, 332.20 to 468.73 and 1.60 to 2.59, for ND, IBD and AI titers, respectively. For ND titer, all three levels of GPC have shown improvement, for IBD, the best level of inclusion found to be in GPC3 group, and other two level had a lesser values and for AI titer, GPC3 and GPC2 levels found to be significantly (p<0.05) higher than GPC1 and control group, when compared with their respective control groups.
|
|
|
6. Conclusions
- In conclusion, the studied growth promoter concentrate in broiler chicken could improve the immunity of antibody titers against ND, IBD and AI values, but did not have a direct positive effect on serum biochemical levels. The best level of inclusion in the diet of this mixture of MOS, EO, vitamins and other essential ingredients found to be 0.1% level in the diet.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran. Authors wish to thank Tehran Dane Feed Manufacturing Company Ltd. for supplying the growth promoter concentrate.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML