-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2013; 3(1): 1-4
doi:10.5923/j.als.20130301.01
In-vitro Free Radical Scavenging, Antioxidant and Antibacterial Activity of Azadirachta Indica A. Juss. of Assam
Deka H., Das S., Lahan J. P., Yadav R. N. S.
Centre for Studies in Biotechnology, Dibrugarh University, Dibrugarh, Pin – 786004
Correspondence to: Das S., Centre for Studies in Biotechnology, Dibrugarh University, Dibrugarh, Pin – 786004.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Azadirachta indica A. Juss.is well known for its wide spectrum therapeutic values and it is an inherent part of conventional medicinal system for many centuries in the Indian Subcontinent curing many household ailments. The antibacterial study was carried out taking six strains of pathogenic bacteria and the antioxidant free radical scavenging activity was studied againsthydroxyl radical, lipid peroxidation, nitric oxide and 2, 2-diphenyl-1-picrylhydrazyl (DPPH).The study showed reasonable activity against In case of Gram positive bacteria theantimicrobial properties while its activity was found poor against almost all the Gram negative bacterial test samples as compared to the standard Ofloxacin. The antioxidant activities were found positive in all except against nitric oxide radical. The Azadirachta Indicais found to be a promising antibacterial and antioxidant agent which can be industrially exploited to produce many useful medicinal products.
Keywords: Azadirachta Indica, Antioxidant, Antimicrobial
Cite this paper: Deka H., Das S., Lahan J. P., Yadav R. N. S., In-vitro Free Radical Scavenging, Antioxidant and Antibacterial Activity of Azadirachta Indica A. Juss. of Assam, Advances in Life Sciences, Vol. 3 No. 1, 2013, pp. 1-4. doi: 10.5923/j.als.20130301.01.
Article Outline
1. Introduction
- Azadirachtaindica A. Juss.Commonly known as Neem is widely distributed in Indian subcontinent and its neighbouring countries for more than a thousand years as one of the most versatile medicinal plant having wide spectrum biological activity. It has been an inherent part of folklore and the traditional medicinal system since one can recall. Every part of the tree is used as medicine for household remedies against various human ailments and even as bio-pesticides for agricultural purpose. Tender shoots and leaves are taken as food in India; in Assam the tiny leaves were oil fried to eat with the rice in early summer like in the month of March-April, which function as an appetizer. The twigs are often used to clean the teeth.Neem oil, leaves, bark, stem products have been therapeutically used for the treatment of respiratory disorder, inflammation, constipation, skin infection[1] arthritic disorders, fever and diabetes etc.[2].Beside these there are several reports on pharmaceutical and biological activities of Neem based on the scientific investigation such as antibacterial, antiviral, antifungal, antioxidant activitiesetc.[3].But due to the varied geographical distribution of the plant a large variety of morphological andbiochemical characteristics can be observed[4] and it has also been re-ported that biological properties of neem is influenced by particular geographical locations and other abiotic factors [5-6]. Therefore, taking account of these reports we have tried to analyse the bioactive compounds, antibacterial and antioxidant properties of Azadirachta Indica A. Juss. of Assam; as it may be a age old customary medicinal plant from myths to households in this region but still lack a proper scientific documentation based on this climatic zone.
2. Methods and Materials
- The healthy leaves of Azadirachta IndicaA. Juss (Family-Meliaceae) had been collected from six different areas of Assam such as Guwahati[North(N) - 26°11, East (E)-91°44′, Altitude (A) – 55.5 m], Tezpur [ N- 26.63°, E-92.8°, A- 48m], Tinsukia[N- 27.500°, E- 95.367°, A – 116m], Silchar[N- 24-82° E- 92-8° A- 22 m]and Dhemaji [N-27°-28°, E- 94°-96°, A- 104 m] in the month of April. The plant was identified in the Department of Life Sciences, Dibrugarh University and a herbarium (No. H-2010/01) was preserved in the laboratory for further references.
2.1. Preparation of Plant Extract
- Extract was prepared from the dry leaves of the A.indica(A. Juss). The coarse powdered obtained from above were used for successive crude extraction by different solvents such as petroleum ether and methanol using Soxhlet apparatus.
2.2. Qualitative Phytochemical Analysis
- Phytochemical screening of plant extracts was done for the presence of alkaloids, quinines, resins, tannins, flavonoids, fats, saponins, phenolic compounds, Proteins and carboxylic acidsaccording to the standard procedure[7-8].
2.3. Antimicrobial Susceptibility Testing
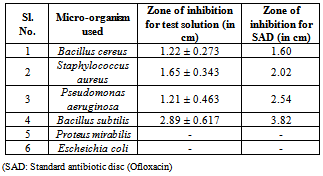
- Antimicrobial susceptibility testing was performed by determining the minimal inhibitory concentration (MIC) and the zone of inhibition. The test was performed by standard broth dilution technique recommended by European Committee for antimicrobial susceptibility testing (EUCAST, E. Dif 3.1.), and the zone of inhibition was determined byKirby-Bauer disc diffusion method[9].For the susceptibility testing three Gram-positive and three Gram-negative pathogenic strains of bacteria were selected for in vitro antibacterial screening. These strains were procured from National Chemical Laboratory, Pune. Gram-positive organisms:Bacillussubtilis (ATCC 11774, NCIM 2063), Bacillus cereus (ATCC 10876, NCIM 2156), Staphylococcus aureus(ATCC BAA 1026, NCIM 2079). Gram-negative organisms: Escherichia coli (ATCC 10536, NCIM 2065), Proteus mirabilis (ATCC 12453), Pseudomonas aeruginosa(ATCC 10662, NCIM 2036).
2.3.1. Turbidity Standard for Inoculum Preparation
- To standardize the inoculum density for a susceptibility test, a BaSO4 turbidity standard, equivalent to a 0.5 McFarland standard was used , prepared by adding a 0.5-ml of 0.048 mol.L-1 BaCl2 (1.175% w/v BaCl2 .2H2O) to 99.5 ml of 0.18 mol/L H2SO4 (1% v/v) with constant stirring to maintain a suspension. McFarland regent was standardized by measuring absorbance at 625 nm and standard absorbance is 0.091.
2.3.2. Inoculation and Incubation
- 1) Sterilized Whatman filter paper discs (0.6 mm), impregnated with the test solution of two different concentrations (256 and 512 μg/ml) were placed on the Muller-Hinton Agar(Himedia, India) plate having the respective test strain. Similarly standard antibiotic discs (Ofloxacin; Sigma-Aldrich, India) were placed on the surface of the medium in the plates as positive controland Dimethyl sulfoxide (DMSO) as negative control and incubated at 370 C for 24 hours.
2.4. Study of Antioxidant and Scavenging Activity
2.4.1. Determination of Hydroxyl Radical (OH -) Scavenging Activity
- Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and methanolic extracts for hydroxyl radical generated by Fenton reaction according to the method of Kunchandy and Rao[10]. Absorbance was measured at 532nm against control containing deoxyribose and buffer. Ascorbic acid was used as a positive control.The hydroxyl radical scavenging activity of the extract is reported as percentage of inhibition (%) of deoxyribose degradation and is calculated as:% OH- radical Scavenging activity= (A Control – A test) / AControl(A Control is the absorbance of the control reaction and Atest is the absorbance in the presence of the sample of the extracts. The antioxidant activity of the extract was expressed as IC50. The IC50 value was defined as the concentration (in μg/ml) of extracts that inhibits the formation of OH- radicals by 50 %.)
2.4.2. Lipid Peroxidation Inhibitory Activity
- Egg yolk was separated and washed with acetone until the yellow colour was removed that contains lectin.. Lipid peroxidation was induced by adding ferric chloride 10μl (400 mM) and L-ascorbic acid 10 μl (400 mM) to a mixture containing egg lectin (3mg/ml) in phosphate buffer solution (PBS) and different concentrations of the extracts (100 μl). After incubation for 1 hr at 37ºC, the reaction was stopped by adding 2 ml of 0.25 N hydrochloric acid containing 15% w/v trichloroacetic acid and 0.375% w/v thiobarbituric acid, boiled for 15 min, cooled, centrifuged, and absorbance of the supernatant was measured at 532 nm.
2.4.3. Determination of DPPH Radical Scavenging Activity
- The free radical scavenging activity of methanolic extract of A. indica (MEAI) was measured by DPPH (1,1-diphenyl-2-picryl-hydrazyl) method described by Blois[11].1 ml of 0.1 mM solution of DPPH in ethanol was added to 1 ml of Methanolic extract of the test sample and after 30 mins of incubation, absorbance was measured at 517 nm. The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples.Ascorbic acid was used as a reference compound. The capability to scavenge the DPPH radical was calculated using the following equation: % DPPH scavenging activity=(A Control – A test) / A Control
2.4.4. Determination of Total Phenolic Compounds
- The total concentrations of phenolic compound present in methanolic extract of A. indica (MEAI) were determined according to the method described bySingletonet al.[12]. 0.1 ml of each extract was transferred to a 100 ml Erlenmeyer flask, and then the final volume was adjusted to 46 ml by the addition of distilled water and then 1 ml of Folin-Ciocalteu reactive was added into this mixture and allowed to stand for 3min then 3 ml of sodium carbonate (2%) was added. Subsequently, the mixture was shaken on a shaker for 2 hrs at room temperature andabsorbance was measured at 760 nm. Pyrocatechol was used as the standard for the calibration curve.The phenolic compound content was determined as pyrocatechol equivalents using the following linear equation based on the calibration curve: A = 0.0034C - 0.058, R2 = 0.9996; where A is the absorbance, and C is pyrocatechol equivalents (μg).
2.4.5. Determination of Total Flavonoids
- Total flavonoid content was determined using the Dowd method as adapted by Arvouet-Grand et al.[13]. Briefly, 1 ml of 2% aluminium trichloride (AlCl3) in methanol was mixed with the same volume of the methanolic extracts. Absorption was taken at 415 nm. The concentrations of flavonoid compounds were calculated according to the following equation that was obtained from the standard quercetin graph:Absorbance, A = 0.0228 Rutin(μg)- 0.0054, R2 = 0.9979
3. Results and Disscussion
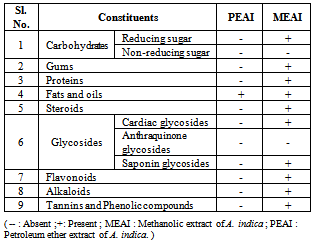
3.1. Qualitative Phytochemical Tests
- The methanolic and ether extracts give varying degree phytochemistry. The methanolic extract showed presence of reducing sugars, gums, proteins, fats, steroids, saponin glycosides, flavonoid, alkaloids, tannins and phenolic compounds. Most of the tests with the petroleum ether extract showed negative results except the presence of fats and oils.
|
3.2. Antibacterial Activity Test
|
3.3. Antioxidant Activity Test
- The Medicinal Plants are known for their antioxidant activity which inhibits the deleterious consequences of oxidative stress. They contain free radical scavengers like polyphenols, flavonoid and phenolic compounds.
3.4. Inhibition of Hydroxyl Radical
- The IC50 value of MEAI was found to be 126.25 ±2.36 (μg/ml) compared to 110.50μg/ml of that of the standard.
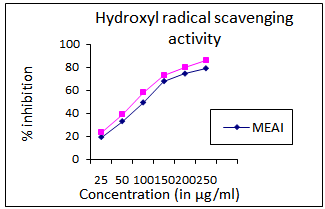 | Figure 1. Hydroxyl radical scavenging activity of methanolic extract of A. indica (MEAI) |
3.5. Lipid Peroxidation Inhibition Activity
- The IC50 of methanolic extract was found to be 179.15 ± 3.21μg/mlandcompared with150 μg/ml of the standard control.
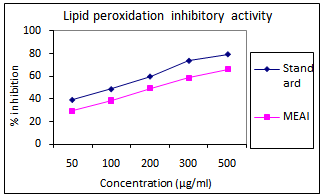 | Figure 2. Lipid peroxidation inhibition activity of methanolic extract of A. indica (MEAI) |
3.6. Inhibition of DPPH Radical
- The IC50 value for methanolic extract was found 150 ± 2.58μg/ml compared to that of standard (169.50 μg/ml). (Fig. 3)
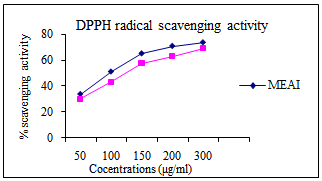 | Figure 3. Inhibition of DPPH radical of Methanolic extract of A. indica |
3.7. Estimation of Total Phenolic and Flavonoid
- The total phenolic and flavonoid content in the methanolic extract (MEAI) of A. Indicawas found 72.38± 2.38μg/mg, 154.89 ± 3.43 respectively.
4. Conclusions
- The study showed that methanolic extract of Azadirachtaindicaleaves is an effective antibacterial agent against various known gram positive and gram negative pathogenic microbes likeBacillus cereus, Staphylococcus aureus, Pseudomonasaeruginosa, Bacillussubtilis, Proteus mirabilis, Escheichia coli, which creates serious health hazards to the human. Andthe leaf extracts is also found to have a significantantioxidant property, which can be industrially exploited to produce many useful pharmaceutical products to tackle the serious damaged caused by free radicals.
ACKNOWLEDGEMENTS
- I would like to express my heartily gratitude to all those who gave us the possibility to complete the above research work. I would like to thank the Department of Pharmaceutical Science and Department of Life-Science for their support and help during the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML