-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(4): 108-111
doi:10.5923/j.als.20120204.05
In Vitro Production of Cucurbitacins From Trichosanthes cucumerina L. var. cucumerina
Devendra N. K1, Everaldo G. Attard2, Raghunandan D3, Seetharam Y. N1
1Plant systematics and Medicinal Plant Laboratory Department of P.G. Studies and research in Botany Gulbarga University, GULBARGA-585 106, INDIA
2Institute of Agriculture, University of Malta, Msida MSD06, MALTA
3H.K.E’s Matoshree Taradevi Institute of Pharmaceutical Sciences, Sedam Road, Gulbarga, Karnataka, India
Correspondence to: Devendra N. K, Plant systematics and Medicinal Plant Laboratory Department of P.G. Studies and research in Botany Gulbarga University, GULBARGA-585 106, INDIA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The objectives of this study were to investigate the effect of growth regulators in callus induction, increase of biomass and to the yield more of cucurbitacin and cucurbitacin-E in leaf explants of Trichosanthes cucumerina L. var. cucumerina. The explants were cultured on Murashige and Skoog (MS) medium supplemented with different concentrations and combinations of auxins like 2,4-dichlorophenoxy acetic acid (2,4-D), [alpha]-naphthalene acetic acid (NAA), Indole butyric acid (IBA), Indole acetic acid (IAA), cytokinins like Kinetin (kn) and Benzyl adenine (BA). The different concentrations and combinations of BAP + IBA, 2, 4-D + BAP and 2, 4-D + kn increased the callus of fresh weight and dry weight. Among all concentrations and combinations, the best results revealed that leaf-derived callus cultured on 2,4-D (3.0mg-l) + kin (1.0 mg-l) produced the highest total cucurbitacins content with an optimum yield 4.9% w/w and cucurbitacin-E 2.75% w/w at third week.
Keywords: Trichosanthes Cucumerina L. Var. Cucumerina, Cell Culture, Cucurbitacins
Cite this paper: Devendra N. K, Everaldo G. Attard, Raghunandan D, Seetharam Y. N, In Vitro Production of Cucurbitacins From Trichosanthes cucumerina L. var. cucumerina, Advances in Life Sciences, Vol. 2 No. 4, 2012, pp. 108-111. doi: 10.5923/j.als.20120204.05.
1. Introduction
- The plant Trichosanthes cucumerina L. var. cucumerina (in English Chinese cucumber or wild snake gourd) belongs to family cucurbitaceae. The fruit is known to contain many form of cucurbitacins, (1, 2, 3) and due to its bitterness the fruit is been using in many ayurvedic preparations (4) and also in Indian folk medicine to cure jaundice (5), to reduce congestion on congestive cardiac failure (6). These days many people are interested in the beneficial effects of food on health, and cucurbitacins have been studied because of the wide range of biological activities they exhibit in living beings. They are predominantly found in the Cucurbitaceae and several other families of the plant kingdom. A number of compounds of this group have been investigated for their cytotoxic (7), hepatoprotective (8), cardiovascular (9), antidiabetic (10) Antibacterial (11), anti- inflammatory (12-13) and antioxidant activity of cucurbitacins B and I and the glucosides of cucurbitacin I and L (14). Additionally, several studies indicated that different cucurbitacin species inhibit the proliferation of cancer cells through different mechanisms (15-19)The members of cucurbitaceae has gained increasing attention as a natural insecticide and its activity has been evaluated against many economically important insect species. Cucurbitta spp. are deterrent, antifeedant, growth- regulating and fertility – reducing properties on insects (20-21) Also, it is used as an abortifacient, cathartic, purgative and vermifuse, and for the treatment of fever, cancer, amenorrhea, jaundice, leukemia, rheumatism, tumour and as an insect repellant (22).The content of cucurbitacins in various organs of Trichosanthes cucumerina L. var. cucumerina has been investigated (23).The present study was carried out to develop an efficient protocol for callus induction, proliferation, total cucurbitacins and cucurbitacin-E accumulation under the effect of different growth hormones with leaf explant of Trichosanthes cucumerina L. var. cucumerina to study various pharmacological effects.
2. Materials and Methods
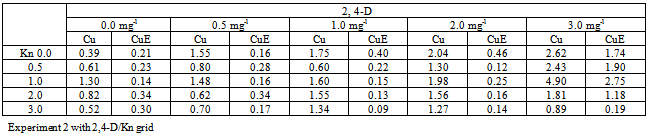
- Quantitative determination of cucurbitacins from in vitro callus culture:Plant materialTrichosanthes cucumerina L. var. cucumerina seeds were obtained from mature fruits collected from Khanapur forest Bhalki taluka, Bidar District India. The collected seed materials were botanically authenticated by the Botany department, Gulbarga University, Gulbarga (Voucher No. HGUG-804). Also, the plant was confirmed with authenticated herbariums at the Centre for Ecological Studies, IISc, Bangalore, and Botanical Survey of India, Pune. The collected seeds preserved in amber bottles under normal lab conditions until used.Media preparationThe basal medium described by Murashige and Skoog (MS) (24) was used. Deferent concentrations of plant growth regulators were added to the MS medium. The media were sterilized by autoclaving at 121℃ for 15 min.Callus inductionThe seeds were surface sterilized with 2% mercuric chloride for 1 min. then washed thrice with distilled water and soaked for 24 hrs in a beaker. The sterilized seeds were used for germination on MS hormone free medium. After few days, the seedlings were excised to yield explants for callus production. The initiated callus was then maintained on MS medium supplemented with BAP 1.0mg-l and IBA 0.5mg-l at 24±2℃ in continuous light (2400 lux) and maintained by transferring approximately 1 g of callus every 4 weeks.Callus propagationFirst experimentApproximately 1 g of initiated callus material was cultured, on different media, to select the best plant growth regulator combination.Second experiment1-1.5 g aliquots of callus were cultured in conical flask containing 100 ml MS medium supplemented with Kn/2,4-D combinations with hormone concentrations of 0.0, 0.5, 1.0, 2.0 and 3.0 mg-l to determine the best callus proliferation and yield of cucurbitacins and cucurbitacin E.Fresh and dry weight measurementFor the first experiment the callus samples were sacrificed on week 3, while for the second one, samples were collected at weekly intervals for a maximum of 5 weeks. After obtaining the fresh weights, the samples were then dried at 40℃ and the dry weights obtained after 24 h.Determination of cucurbitacinsSolvents and reagentsAbsolute ethanol, petroleum ether 30-40℃, chloroform and phosphomolybdic acid (all at analar grade). A cucurbitacin E reference standard was used.Sample solutionsFor total cucurbitacin assay, dried callus material (100-200 mg per sample) was extracted with absolute ethanol (5ml) for 2 h, after centrifugation (2000 rpm for 3 min), the supernatant was mixed with an equal volume of petroleum ether, the precipitate obtained was filtered and dissolved in absolute ethanol (5ml), and then reduced to a volume of 2 ml as above.Reference solutionThe reference standard cucurbitacin E was dissolved in ethanol and serial dilutions (0.01-1.0 mg/ml) were prepared.AsseyAll samples (100μl, in duplicate), together with various concentrations of cucurbitacin E standard as per (25) at room temperature. The absorbance was measured at 492 nm after 5 min on a MTP reader STATFAX2100, USA. The results were expressed as w/w% calculated from dry callus weight and then analyzed statistically by ANOVA.
3. Results and Discussion
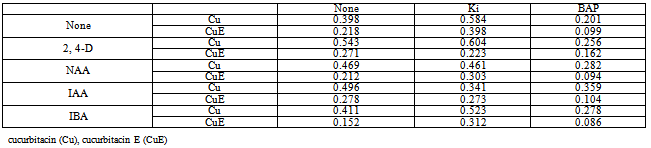
- Quantitative determination cucurbitacins from in vitro callus culture:Experiment 1 with mixed PGRs gridBiomass accumulation:Table-1 indicated that BAP was the best cytokinin in combination with IBA as regards callus accumulation. Calluses on these plant growth regulators were friable and white in colour, while with kinetin and 2,4-D, these were relatively hard and brown in colour, and showed a slow rate of accumulation. Secondary metabolite accumulation:On other hand 2,4-D and Kn gave the most significant Cu and CuE accumulation, especially when compared to IBA and BAP (Table-2). Overall, an inverse proportionality was observed between callus weight and cucurbitacin yield. This was clearly shown in the combination of 2,4-D and IBA with BAP, having dry weights of 187 and 258 mg, respectively, and corresponding Cu contents of 0.359 and 0.256% w/w. similar results were observed in Ecballium elaterium (26).
|
|
|
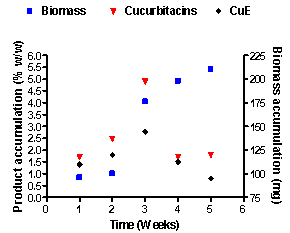 | Figure 1. The pattern of growth linked accumulation of cucurbitacins (Cu) and cucurbitacin E (CuE) in T. cucumerina L. var. cucumerina (treatment: 2,4-D 3.0mg-l + Kn 1.0mg-l |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML