-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(4): 98-103
doi: 10.5923/j.als.20120204.03
Greenhouse Biological Approach for Controlling Foliar Diseases of Some Vegetables
M. M. Abdel-Kader , N. S. El-Mougy , M. D. E. Aly , S. M. Lashin , F. Abdel-Kareem
Plant Pathology Department, National Research Centre, Dokki 12622, Giza, Egypt
Correspondence to: N. S. El-Mougy , Plant Pathology Department, National Research Centre, Dokki 12622, Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Evaluation the efficacy of bio-agents, application as foliar spray against vegetables foliar diseases incidence was carried out in open greenhouse conditions. The tested bio-agents were Trichoderma harzianum, T. Viride, Bacillus subtilis, Pseudomonas fluorescens and Saccharomyces cerevisiae were evaluated. The recorded foliar diseases, i.e. Powdery, Downy mildews of Cucumber, Cantaloupe and Pepper as well as Early, Late blights of Tomato were significantly reduced at all treatments either alone or in combinations comparing with untreated plants. Application with either T. harzianum and B. subtilis showed significant reduction in diseases incidence comparing with the other applied bio-agents. The other bio-agent treatments, T. viride, P. fluorescens and S. cerevisiae recorded moderate reduction in this concern. On the light of the present study it could be suggested that the usage of bio-agents might be used as easily applied, safely and cost effective control methods against such foliar plant diseases.
Keywords: Cucumber, Cantaloupe, Pepper, Tomato, Bio-Agents, Powdery Mildew, Downy Mildew, Early Blight, Late Blight
Article Outline
1. Introduction
- Vegetable crops grown under protected cultivation, which considered as Egypt’s largest export national income, facing a serious problem due to diseases infection that cause about 20-30 % loss of produced yield because of favourable environment for disease incidence and development. Therefore, many control practices need to be integrated in order for minimizing this figure to occur. Powdery and Downy mildews as well as Early and Late blights are the most serious foliar diseases attacked vegetables grown in plastic houses. Powdery mildew disease is one of the most serious plant diseases, causing large yield losses in a number of crops[1]. Powdery mildew, is a serious disease affecting the leaves, stems and fruits of cucumber (Cucumis sativus L.) grown in greenhouses and in the field[2]. Powdery mildew of cucumber is one of the most dangerous foliar diseases, attacking cucumber plants in Egypt and other countries[3-5]. Moreover, powdery mildew, caused by Erysiphe cichoracearum DC, is one of the most serious diseases affecting cantaloupe production, with a high severity at the time of fruit maturity[6]. Also, late and early blights of tomato caused Phytophthora infestans and Alternaria solani were also recorded in growing greenhouse tomato[7, 8].In order to overcome such hazardous control strategies, scientists, researchers from all over the world paid moreattention towards the development of alternative methods which are, by definition, safe in the environment, non-toxic to humans and animals and are rapidly biodegradable, one such strategy is use of Biocontrol agents (BCAs) to control fungal plant diseases. Among the BCAs, species of the genus Trichoderma is most promising and effective biocontrol agent.Trichoderma as antagonist controlling wide range of microbes[9]. Inhibition of pathogenic fungi has been reported as a result of BCAs treatment included in powdery mildew (Leveillula taurica) in sweet pepper[10] and powdery mildew in cucumber[11]. Furthermore,[12] recorded the possibilities for biological control of powdery mildew (Sphaerotheca fuliginea, Erysiphe cichoracearum) and mildew (Pseudoperonospora cubensis) on cucumber by Enterobacter cloacae (J.) using the bio-agent Enterobacter cloacae sprays. On the other hand,[13] reported that based on the whole plant tests, foliar spray with Paenibacillus macerans-GC subgroup A, Serratia plymuthica, Bacillus coagulans, Serratia marcescens-GC subgroup A, Bacillus pumilis -GC subgroup B and Pantoea agglomerans bacterial isolates reduced the disease severity of early blight significantly when compared with control. These results suggest that the bacterial isolates studied have a good potential to be used as biocontrol agents of A. solani in tomato.The aim of present study is to evaluate the abilities of foliar spray with different bio-control agents for controlling Powdery and Downy mildews of Cucumber, Cantaloupe, Pepper and Early, Late blights of Tomato under open greenhouse conditions.
2. Materials and Methods
2.1. Plant Materials
- Trasplants of Cucumber (cv. Alpha), Cantaloupe (cv. Yatherb 7), Tomato (cv. Castel Rock) and Pepper (cv. California) were used in the present study.
2.2. Bio-agents
- The tested antagonistic fungi were Trichoderma harzianum, T. Viride and the bacteria Bacillus subtilis, Pseudomonas fluorescens and the yeast Saccharomyces cerevisiae. These antagonists were isolated from the rhizosphere of cucumber, cantaloupe, tomato and pepper grown in plastic houses under protected cultivation systems and showing root rot disease symptoms[14]. The present bio-agents proved their antagonistic ability against the above mentioned pathogens under in vitro conditions.
2.3. Bio-agents Inocula Preparation
- The antagonistic bacteria (B. subtilis, P. fluorescense) were grown on nutrient broth medium while yeast (S. cerevisiae) was grown on NYDB medium[15]. All tested bacteria and yeast incubated in a rotary shaker at 200 rpm for 24 h at 28 ±2℃. The bacterial and yeast cells were harvested by centrifugation at 6,000 rpm for 10 min, washed twice with 0.05 M phosphate buffer at pH 7.0, and re-suspended in sterilized distilled water. The concentrations of both bacteria and yeast cells in the suspensions were adjusted to 1x105-106 cells per millilitre (Cfu / mL) with the aid of a haemacytometer slide. Meanwhile, antagonistic fungi were grown on PDA medium[15] incubated for 72 h at 25 ±2℃. Fungal conidia were harvested by scraping the surface of the colonies with a spatula and transferred to sterilized distilled water and filtered through nylon mesh, then spore suspension was adjusted 1x104-105 spore per millilitre (Cfu / mL) with the aid of a haemacytometer slide.
2.4. Greenhouse Experiments
- Evaluation the efficacy of bio-agents application as foliar spray against vegetables foliar diseases incidence, the following procedures was carried out in open greenhouse, National Research Centre, Egypt. Transplants of Cucumber, Cantaloupe, Tomato and Pepper were planted in natural loamy soil as three transplants per pot and five pots per replicates in each particular foliar treatment. Foliar spraying with tested bio-agents, i.e. T. harzianum, T. viride, B. subtilis, P. fluorescens and S. cerevisiae were applied twice with two weeks intervals starting one week from transplanting. One week after the second antagonistic foliar application, foliar artificial infestations with Sphaerotheca fuliginea and Peronoplasmopara cubensis the causal fungi of Cucumber, Cantaloupe and Pepper Powdery and Downy mildews, respectively as well as Alternaria solani the causal fungi of Tomato Late blight was carried out as spraying of pathogen suspension (2x104cfu/mL). Phytophthora infestans the causal of Tomato Early blight was applied as soil drench through soil irrigation with fungal suspension (2x104cfu/mL) after[16] at the rate of 50ml/Pot.These mentioned pathogenic fungi were obtained from collected diseased vegetables grown in different locations of commercial protected cultivated plastic houses at the same growing season. Fungal suspensions was carried out in distilled water and also adjusted to 1x104-105 spore per millilitre (Cfu / mL) with the aid of a haemacytometer slide.Another set of soil planted with tested vegetables transplants sprayed only with foliar diseases incidents was kept as control check treatment. Appearance of different foliar diseases incidence were recorded periodically and the average accumulated percentages were calculated after 60 days of transplanting, the experimental period.Statistical analysisAll experiments were set up in a complete randomized design. One-way ANOVA was used to analyze differences between applied treatments and disease incidence. A general linear model option of the analysis system SAS[17] was used to perform the ANOVA. Duncan’s multiple range test at P ≤ 0.05 level was used for means separation[18].
3. Results and Discussion
- Results in Table (1) and Fig (1) represented the efficacy of foliar spraying with bio-agents against vegetables foliar diseases incidence under greenhouse conditions. Presented data revealed that Powdery and Downy mildews were detected only on Cucumber, Cantaloupe and Pepper while Early and Late blights were recorded on Tomato plants. Data also showed that all applied bioagents significantly reduced the recorded foliar diseases comparing with untreated control. Application with either T. harzianum and B. subtilis showed significant reduction in diseases incidence comparing with the other applied bio-agents. The recorded percentage of Powdery mildew in bio-agents spray application ranged between 13.3-33.3% in Cucumber, 13.3-30.0% in Cantaloupe and 16.6-33.3% in Pepper comparing with 50.0, 43.3 and 40.0% in control treatment, respectively. The other bio-agent treatments, T. viride, P. fluorescens and S. cerevisiae recorded moderate reduction in Powdery mildew incidence ranged between 20.0-26.6% of the tested vegetables. Similar trend was also observed for Downy mildew in Table (1). The recorded disease incidence were 16.6-30.0% of Cucumber, 13.3-33.3% of Cantaloupe and 16.6-36.6% of Pepper comparing with 53.3, 40.0 and 46.6% in control treatment for sprayed plants in respective order. Tomato plants recorded Early and Late blights infection as 16.6-30.0% and 16.6-33.3%, comparing with unsprayed plants which showed diseases incidence calculated as 33.3% and 36.6% for both diseases, respectively.In this concern several workers conducted with bio- control applications against plant diseases control were reported. Biological control of vegetable foliar diseases by different micro-organisms is a part of important researches over the world. Overall, reduction of pesticide use is one objective, on the other hand the decline of residues on agriculture products is another issue and here the biocontrol of foliar diseases can have a significant effect. Biological control using natural products or antagonistic micro-organisms proved to be successful for controlling various plant pathogens in many countries[19]. It is still not expensive and is easy in application, however it can serve as the best control measure under restricted conditions.In addition, its application is safe, un-hazardous for human and avoids environmental pollution[20]. In this regard, [21] reviewed that during the past ten years, over 80 biocontrol products have been marketed worldwide. A large percentage of these have been developed for greenhouse crops. Products containing Trichoderma, Ampelomyces quisqualis, Bacillus, Ulocladium and Pseudomonas are being developed to control the primary foliar diseases, Botrytis and powdery mildew in greenhouses could predominate over chemical pesticides.In the present study, spraying vegetables, Cucumber, Cantaloupe, tomato and Pepper with the bio-agents, T. harzianum, T. viride, B. subtilis, P. fluorescens and S. cerevisiae was effectively able to reduce the foliar diseases comparing with untreated control. These results were in agreement with several previous reports. A field experiment was conducted at Rahuri, Maharashtra[22], India to investigate the efficacy of the culture filtrates of T. viride, T. harzianum, T. hamatum, T. longiflorum and T. koningii and the fungicide carbendazim against the powdery mildew (Leveillula taurica) of guar. They found that all treatments recorded beneficial effects on growth parameters and disease control. Also, [23] recorded that Trichoderma harzianum which can be regarded as a model to demonstrate biocontrol under commercial conditions and the mechanisms involved. He added that this biocontrol agent controls the foliar pathogens, Botrytis cinerea (gray mold), Pseudoperonospora cubensis (downy mildew), Sclerotinia sclerotiorum (foliar blight) and Sphaerotheca fusca (powdery mildew) in cucumber under commercial greenhouse conditions.Similarly, [24] evaluated culture filtrates of T. viride, T. harzianum, T. hamatum, T. Iongiforum and T. lconlngll for the management of powdery mildew of Cluster bean caused by LevelIlula taurica. They found that culture filtrates of Trichoderma spp. either alone or in combination were found effective against powdery mildew. Also, Trichoderma harzianum T39 (TRICHODEX) spray reduced powdery mildew severity caused by Sphaerotheca fusca on greenhouse cucumber by up to 97%[25,26]. Furthermore, [27] stated that foliar application of Pseudomonas fluorescens combined with a half of the recommended dose of azoxystrobin is of practical significance, since an application of fungicide alone requires three to four further following sprays for an effective control of downy and powdery mildews of cucumber.
|
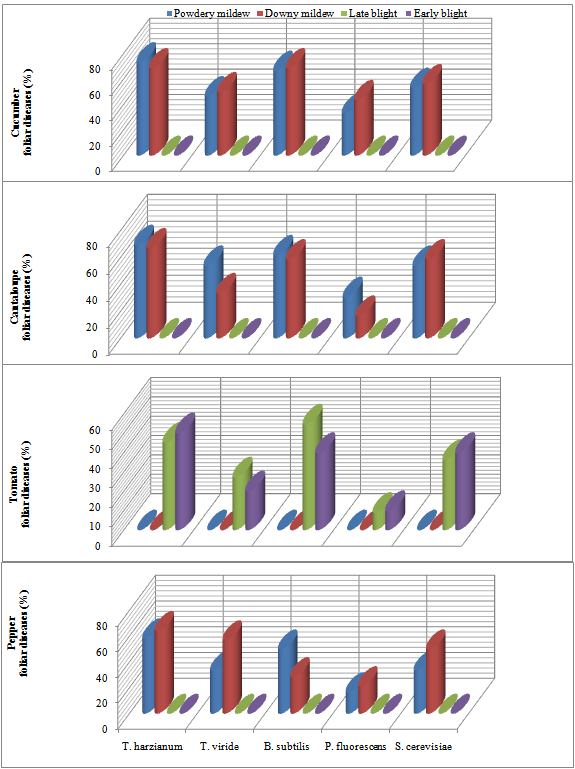 | Figure 1. Reduction in vegetables foliar diseases in response to spraying with antagonistic bio-agents under open greenhouse conditions |
ACKNOWLEDGMENTS
- This work was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No. 1059.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML