-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(3): 75-81
doi:10.5923/j.als.20120203.06
On the Morphological and Genotypic Variations of Two Congeneric species of Banana Aphid Pentalonia (Homoptera: Aphididae) from India
Parna Bhadra, Basant Kumar Agarwala
Ecology and Biodiversity Laboratory, Department of Zoology, Tripura University, Suryamaninagar, 799 022, Tripura, India
Correspondence to: Parna Bhadra, Ecology and Biodiversity Laboratory, Department of Zoology, Tripura University, Suryamaninagar, 799 022, Tripura, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Banana aphid Pentalonia has already been described in its two taxonomic forms from hosts of Zingiberaceae and Araceae were regarded as separate taxa, P. nigronervosa Coquerel and P. caladii van der Goot, respectively, based on morphological and molecular differences. Between the two species of Pentalonia tested in our earlier study, the nigronervosa speciesexpressed fitness for banana host plants and the caladii species for taro host plant suggesting strong genotype(aphids)-environment(host plants) interactions and increased genetic variation. This study shows the morphological variations of the two species and the isozyme variations of the two taxa from Araceae and Musaceae plants, respectively, an indicative of this separation as the laboratory-reared clones of banana aphidfrom the host plants also occur as two variants of the aphid species. In the existing conditions of information, the two species can be certainly considered as separate species.
Keywords: Banana Aphid Clones, Two Taxonomic Forms, Host Plant Induced Variations, Isozymes
Cite this paper: Parna Bhadra, Basant Kumar Agarwala, On the Morphological and Genotypic Variations of Two Congeneric species of Banana Aphid Pentalonia (Homoptera: Aphididae) from India, Advances in Life Sciences, Vol. 2 No. 3, 2012, pp. 75-81. doi: 10.5923/j.als.20120203.06.
Article Outline
1. Introduction
- The banana aphid is generally known to occur by asexual morphs, wingless and winged parthenogenetic viviparous females in the Indian subcontinent[1,2]. However, worldwide, P. nigronervosa is recognized due to its vector populations on banana plants[3-5]. Coquerel (1859) first described P. nigronervosa from banana from the Indian Ocean island of Réunion[6]. Subsequently, a second species P. caladii from Caladium[7] was described from Java, without explicitly mentioning P. nigronervosa or providing characters distinguishing the two. Hardy[8] considered the observed difference between the two Pentalonia species to be environmentally induced and placed P. caladii as synonymy of P. nigronervosa. Eastop[9], however, recognized the distinguishable variation within P. nigronervosa based on taxonomic differences in winged morphs from Australia and some other parts of southern hemisphere and considered it to be represented by two forms, P. nigronervosa f. typica infesting plants of Musaceae and P. nigronervosa f. caladii van der Goot found on plants of Araceae[10,11]. Most of the authors later maintained the taxonomic position of Eastop[9]. Ayyar[12] reported that the aphids found on Colocasia plants in South India belongs to P. caladii. Siddappaji and Reddy[13] reported that the aphids occurring in banana plants in parts of South India belong to the form typica Eastop and those of cardamom and Colocasia sp. belong to the form caladii van der Goot. A few faunal lists have treated these as separate species[14]. The two species, Pentalonia nigronervosa and P. caladii, were both distinguished from Pentalonia kalimpongensis (A.N. Basu 1968)[15] by Noordam[16]. In a recent study based on morphological and molecular differences, the two forms of the banana aphid were given their species status, respectively,[17]. Bhadra and Agarwala[18] further recorded ecological and biological differences between P. caladii and P. nigronervosa from Araceae and Musaceae plants, respectively and considered these as full species. Banana plants are infested by aphids (P. nigronervosa) at the base of the pseudo stem, young suckers, the areas between the sheath of the outer leaf, bases of the uppermost cigar-shaped leaves and in spathe while taro plants are infested by P. caladii on pseudo stem near the root and seldom at the bases of broad open leaves. These host plants co-exist in large areas of east and north-east India, provide different food environments for colonization and, therefore, offer equal opportunities of adaptation to the aphid populations infesting them.In view of our earlier findings, it is assumed that the aphids of the two Pentalonia species from their respective host plants might show other differences that could further substantiate their occurrence as distinct species from India. In this study the two populations of Pentalonia aphids collected from two different host plants viz., Musa paradisiaca L. (banana, local variety – champa, Family Musaceae) and Colocasia esculenta antiquorum (L.) (taro, Family Araceae) were distinguished in terms of their morphological (morphometrical) and electrophoretic (isozyme) variations. Two taxonomic keys, one each to the identification of adult alatae and adult apterae females from the two host plant-specific aphids is provided.
2. Materials and Methods
- Parthenogenetic viviparous apterae of Pentalonia nigronervosa and P. caladii aphids were collected from different taro and banana plants found in the wild at five different locations, separated by about 2000 m distance from each other, in and around Agartala, north-east India (23.50°N latitude and 91.25°E longitude). These aphids were used to raise ten stock cultures, five each from five locations on the two host plants, under greenhouse conditions (24± 1° C temperature and 16: 8 L: D photoperiod). Host plants were maintained in early vegetative stage individually in clay or plastic pots and these were held in water trays on benches illuminated with photo-synthetically active radiation lamps. Individual plants, two from each location, were infected with a single fourth instar apterous aphid collected from their respective locations in the fields. These were allowed to grow, reproduce and increase in number. Aphid cultures on individual potted plants were confined in nylon net cages in segregated locations. This was repeated ten times for each plant species. All aphids produced from a single mother on each of the plants by this practice consisted of the same genotype and, thus, constituted a clone. Fourth instar aphids produced of the same genotype of a grandmother on a plant species were used in experiments. Individual aphids, chosen randomly from banana and taro plants in the greenhouse, were placed on the apical parts of 16-20 day old pot-grown saplings at the early vegetative stage in a rearing cabinet (temperature: 24 ± 1° C; 65% RH and 16: 8 L: D photoperiod). The two host plants used in experiment were: banana local var. champa (Bc) and taro with green petiole (Tg). Thus, several sister clones of the same genetic lineage of the two species were raised on their respective host plants. Aphid-infected individual plants were individually caged to avoid any contamination during the experiment and plants of the two hosts were substituted for any that had deteriorated. This practice allowed an uninterrupted supply of aphids from the two host plants. Parthenogenetic wingless (apterae) female aphids were used for sample preparation for isozyme study while parthenogenetic winged (alate) and wingless (apterae) females were used for morphological and morphometric studies.
2.1. Morphometric Parameters
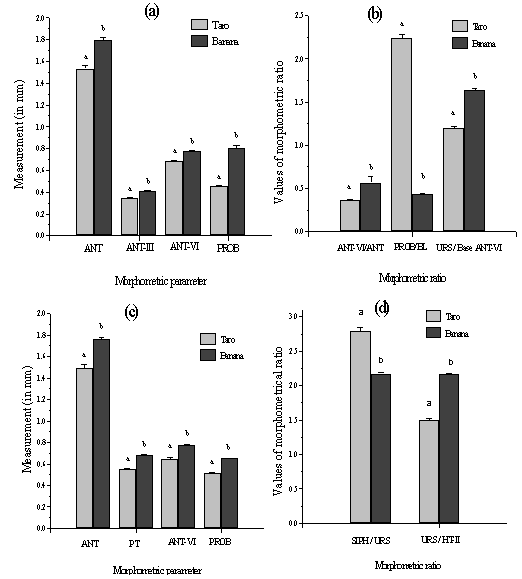
- Adult alate and apterous aphids chosen randomly from banana plants and taro plants in the field were used to record variations in morphometry. Aphids were prepared for microscopic examination of whole mounted specimens following the method of Raychaudhuri[1]. Thirteen characters (fourteen characters in alates) of taxonomic importance from each aphid specimen were measured using an eye piece micrometer : length of body (BL), width of body (BW), length of antenna (ANT), third antennal segment (ANT III), base of sixth antennal segment (Base VI), processus terminalis of sixth antennal segment (PT), antennal segment VI (ANT VI), proboscis (PROB), ultimate rostral segments (URS), siphunculus (SIPH), least diameter of siphunculus (LD-SIPH), length of cauda (CAU), hind tarsal segment II (HT-II) and length of forewing (in alate) (FW). In addition, ratio of length of body to length of antenna (BL/AL), length of base of ANT VI to processus terminalis (Base VI/PT), length of body and proboscis (BL/PROB), length of URS to proboscis (URS/PROB), length of Siphunculus to its least diameter (SIPH/LD-SIPH), length of siphunculus to URS (SIPH/URS), URS to hind tarsal segment II (URS/HT-II) and length of URS to base of ANT VI (URS/BaseVI) were also determined from these aphids.
2.2. Morphology
- Pigmentation pattern of the siphunculi was observed to distinguish the two species collected from their respective host plants.
2.3. Electrophoretic Studies
- i) Sample and gel preparation200 mg of apterous aphids were obtained from each of the host-specific clones (all individuals of a sample were identical genotypes of one parthenogenetic mother). 15% homogenized sample solution in a 1:1 mixture of sucrose and 0.1 M TRIS-HCl extraction buffer (pH 6.8) was centrifuged (10,000 rpm; 20 min; – 6 oC) and the supernatant was stored at – 4 oC after thoroughly mixing 0.2% (w/v) bromophenol blue to it as a front-running dye. About 100 µg of proteins of each sample contained in a mixture was loaded onto a polyacrylamide slab gel pre-soaked in electrode buffer (3.035 g TRIS-HCl, 14.4 g glycine at pH 8.3), using a 7-lane vertical electrophoretor. Gels were generally run for two hours in constant current at 12 mA per gel (6.5 cm). 10 ml of 8% resolving gel (2.7 ml of 30% acrylamide and 0.8% bis-acrylamide solution, 2.5 ml of 1.5 M TRIS-HCl buffer-pH 8.8, 4.7 ml double distilled water, 0.006 ml TEMED, and 0.1 ml of 10% ammonium persulphate solution added in sequence) and 5 ml of 4.5% stacking gel (0.83 ml of 30% acrylamide and 0.8% bis-acrylamide in 0.63 ml of 0.5M TRIS-HCl buffer-pH 6.8, 3.45 ml double distilled water, 0.005 ml TEMED, and 0.05 ml 10% APS added in sequence) were prepared. ii) Preparation of enzyme buffers and staining mixtureEsterase: 100 ml of 0.1 M Na-phosphate buffer contained 1.21% NaH2PO4.2H2O and 0.28% Na2HPO4 anhydrous at pH 6.0; Malic dehydrogenase: 50 ml of 0.1M TRIS-HCl buffer at pH 8.5; Acid phosphatase: 100 ml of 0.5 m acetate buffer contained 9.3% glacial acetic acid and 0.5% NaOH at pH 5.0. Staining mixtures were prepared according to the procedure of Loxdale et al.[19] and Singh & Cunningham[20]. (Esterase: 50 ml of enzyme buffer, 50 mg substrate-naphthyl acetate, 0.6 ml acetone, 0.6 ml distilled water and 50 mg of Fast Garnet GBC salt stain; Malic dehydrogenase: 7.5 ml of 0.1M TRIS-HCl buffer, 62.5 ml double distilled water, 15 mg DL-malic acid, 10 mg NAD, 5 mg NBT and 10 mg PMS; Acid phosphatase: 50 ml of enzyme buffer, 60 mg α-naphthyl acid phosphate and 60 mg of Fast Garnet GBC salt stain). Staining filtrate were stored at <5 oC except for malic dehydrogenase (37 oC).iii) Mobility of enzymes and gel analysisGels were, at first, kept in respective enzyme buffer solutions for 40 min (Esterase and acid phosphatase at <5 oC; malic dehydrogenase at 37 oC). Subsequently, gels were incubated in respective reaction mixtures at 37 oC (esterase = 30 minutes, malic dehydrogenase = 4 hrs, acid phosphatase = 2 hrs). Relative migration was determine as Rm = distance migrated by specific bands (mm)/distance migrated by marker (mm). A comparison of host plant related inter-clonal variations observed in the gels was done. Homogenates of different clones were used in the same run and the relative separation distances of different isoenzyme bands of a given enzyme were obtained in relation to the front running dye. The number of bands and the Rm of each band were used as indicators of genetic similarity and difference between the two aphid species.
3. Results
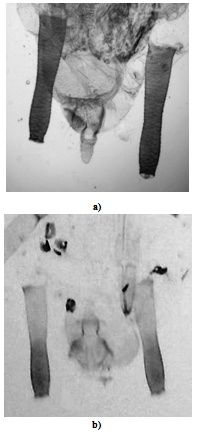
3.1. Morphological and Morphometrical Variations
- Alate morph Adult apterous aphids of P. nigronervosa from the banana plants had longer proboscis, longer ultimate rostral segments and longer antennae, sixth antennal segment in particular, than in the adult apterous aphids of P. caladii from the taro plants. For rest of the morphometrical variables such as BL, BW, LD-SIPH, CAU and HT-II, significant differences in mean values were recorded between the aphids from banana and taro plants but the minimum and maximum ranges of these characters did not show distinguishable variations i.e., these values overlapped. Significant differences between the aphids of two species (i.e., from the two plants) were also recorded in the ratio of BL/PROB, URS/SIPH, URS/HT-II and URS/Base ANT-VI. Likewise, the aphids from the taro plants had SIPH 2.50-3.20 times URS which is 1.428-1.833 times HT-II and 1.00-1.33 times Base ANT-VI, respectively, in comparison to SIPH 1.928-2.50 times URS which is 1.75-2.33 times HT-II and 1.50-1.75 times Base ANT-VI, respectively, in aphids from the banana plants. Apterae morphAphids from the taro clones had siphunculi constriction in the middle on inner margin alone and are uniformly pigmented brown throughout the length (Fig. 2a). In comparison, in aphids from the banana clones, siphunculi showed distinct constrictions in the middle on both inner and outer margins and also showed paler pigmentation in the basal region and darker brown pigmentation distally (Fig. 2b).Results showed that aphids from the banana clone had longer proboscis, longer ultimate rostral segments and longer antennae, sixth antennal segment in particular, than in aphids from the taro clones. In other morphometrical variables such as BL, BW, LD-SIPH, CAU and HT-II also showed significant differences between the clones from banana and taro plants but the minimum and maximum ranges of these characters overlapped. Significant differences between the two clones were recorded in the ratio of BL/PROB, URS/SIPH, URS/HT-II and URS/Base ANT-VI. Thus, adult apterae from the taro clones have proboscis 0.33-0.44 times body in comparison to 0.43-0.52 times body length to proboscis of aphids from banana clones. Likewise aphids from taro clones have URS 2.46-3.18 times SIPH, 1.12-1.86 times HT-II and 0.90-1.30 times Base ANT-VI respectively in comparison to 1.94-2.33 times SIPH, 1.92-2.34 times HT-II and 1.36-1.66 times Base ANT-VI respectively in aphids from banana clones.The alate and apterous viviparous morphs of the two aphid species from the respective host plants can be distinguished by the following key characters:A. Key to the separation of caladii and nigronervosa species of Pentalonia based on alate morphsSiphunculi uniformly pigmented (Fig.2a), 2.5-3.2x as long as URS which is 1.43-1.83x as long as hind tarsus-II and 1.0-1.33x as long as base antennal segment VI; proboscis 0.42-0.51 mm long, 0.338-0.391x as long as body; antennae 1.24-1.64 mm long, 1.01-1.35x the length of body; colonise arum plants (Colocasia esculenta antiquorum)Pentalonia caladiiSiphunculi often paler near the base (Fig. 2b), 1.93-2.5x as long as URS which is 1.75-2.33x as long as hind tarsus-II and 1.5-1.75x as long as base antennal segment VI; Proboscis 0.75-0.9 mm long; 0.503-0.625x as long as body; antennae 1.66-1.91 mm long, 1.12-1.36x the length of body; colonise banana plants (Musa paradisiaca var. champa )P. nigronervosa
3.2. Electrophoretic Variations
- Pentalonia aphids were found to be highly polymorphic for esterase and less so for the other two enzymes (table 1). Two sets of esterase isozymes were identified from the four host plants. These comprised of eleven distinguishable bands (Fig. 3 a), Est-1 to Est-11, in the order of their increasing mobility. Band at positions Est-3 and Est-5, prominent in the aphids from taro were absent in the aphids from banana plants. Est-6 was common to both the aphid clones. A moderately-fast-moving band at Est-9 (Rm: 0.676) was unique to banana champa aphid clones. Est-10 and Est-11 was common to all the aphid clones.
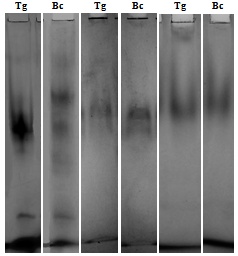 | Figure 3. Isoenzymatic patterns in P. nigronervosa aphids. Esterase pattern in relation to host plants—1st and 2nd lanes from left : Tg = aphid clones from taro plants with green petiole, Bc = aphid clones from banana var. champa, Malic dehydrogenase pattern in relation to host plants— 3rd and 4th lanes from left : Tg-Bc and Acid phosphatase pattern in relation to host plants—5th and 6th lanes from left : Tg-Bc |
4. Conclusions
- Previous studies distinguished the two forms (later distinguished as two species of Pentalonia) of banana aphid on the basis of morphological difference in winged viviparous morph[9]. This study has shown that the winged and wingless viviparous morph of the two species also show consistent difference in morphology, particularly in the feeding characters (lengths of proboscis and ultimate rostral segments) and sensory character (length of antennae) which are of immense adaptive value. The differences in band patterns of the three enzymes in this study suggested genetic differences between Pentalonia nigronervosa and P. caladii from banana and taro host plants. The three enzymes used in this study fell into two categories. Acid phosphatase from the two species as well as malic dehydrogenases from the two species showed almost no difference in band mobility, thus, considered as comparatively less suitable for taxonomic studies. Esterases, which were represented by multiple loci, showed variation in intensity and mobility of bands in different clones from the two aphid species, are taxonomically more important enzymes. Esterase was observed to be the most variable in the aphids from the studied host plants. Est-2, Est-4, Est-9 and Est-10 appears to be the most distinct among all bands obtained in the electrophoretograms of the two aphid species.Pentalonia are commonly known by their winged and wingless viviparous morphs and reproduce by asexual means in the environment of north-east India and elsewhere in their distribution range of tropical and subtropical regions[9],[1],[21],[17]. Aphids from the Neoarctic and Palearctic regions belong to sexually-reproducing species which are characterized by liberal gene flow between populations from different host plants. In contrast, asexual population of aphids in the hotter parts of Oriental and African regions lack gene flow, and the observed host-specializations in polyphagous and oligophagous species have not been adequately explained. A number of studies of insect herbivores have found significant intraspecific variation in characters associated with host plant utilization[22,23]. It has been shown that intraspecific variation can be caused either by genetic differences or effects of experience on tested host on tested host plants[24-26].The results of this study give additional weight to the typica/caladii distinction of the earlier studies or the nigronervosa/caladii distinction (sensu Foottit et al., 2010; Bhadra and Agarwala, 2010 ) of existing studies, and also provide further evidence that the taro and banana-adapted genotypes retain their distinct morphology and isozyme mobility, although in parthenogenetic rearings. Furthermore, the banana and taro-adapted genotypes (if considered so) have retained their morphological identity in another region i.e., India, apart from Java[7], Australia[9], Countries of southern hemisphere[10] and parts of tropical and sub-tropical regions[17]. The two earlier considered genotypes or forms can thus be easily considered as two species P. nigronervosa and P. caladii on the basis of their morphological, biological and electrophoretic variations. Between the two species of Pentalonia tested in this study, the typica species expressed lower fitness on banana host plant than the caladii species from taro host plant[18]. Such a difference in response to host plants is suggestive of strong genotype (aphids)-environment (host plants) interactions indicating increased genetic variation. In some way these genotypes (here considered as two species) were able to maintain a coherent metabolic integration from one environment to another without substantially compromising their fitness output[27]. The hypothesis of sympatric speciation in phytophagous insects occurring via phenotypic host race formation has been gaining acceptance in recent years[28-31] and explains how phenotypic plasticity facilitates speciation[32,33]. In view of the results of this study, it would be interesting to know whether P. nigronervosa populations reported from cardamom, ginger, Heliconia spp., Caladium spp., Alpinia spp. and Dieffenbachia spp. host plants[2] belong to any of the two species distinguished here or to some unknown species or new forms.
ACKNOWLEDGEMENTS
- Authors acknowledge to the Indian Council of Agricultural Research (ICAR), New Delhi for partial resource support.
References
| [1] | D. N. Raychaudhuri (ed.) “Food plant Catalogue of Indian Aphididae”, The Aphidological Society, Calcutta (India). 1-203, 1980. |
| [2] | Roger L. Blackman, V. F. Eastop, “Aphids on the World’s Crops: An Identification Guide”, John Wiley, New York, 1984. |
| [3] | P. Rajan, “Biology of Pentalonia nigronervosa F. caladii van der Goot, vector of ‘katte’ disease of cardamom”, Journal of Plantation Crops, vol. 9, pp.34–41, 1981. |
| [4] | J. S. Hu, M. Wang, D. Sether, W. Xie, K. W. Leonhardt, “Use of polymerase chain reaction (PCR) to study transmission of banana bunchy top virus by the banana aphid (Pentalonia nigronervosa)”, Annals of Applied Biology, vol.128, pp.55-64, 1996. |
| [5] | G. Thinbhuvanamala, S. Doraiswami, T. Ganapathy, “Detection of BVTV in the aphid vector using (DAS)-ELISA”, Indian Journal of Virology, vol.16, pp.12-14, 2005. |
| [6] | C. Coquerel, “Note sur quelques insectes de Madagascar et de Bourbon”, Annales de la Société Entomologique de France (3ième séries), vol.7, pp.239–260, 1859. |
| [7] | P. van der Goot, “Zur Kenntnis der Blattläuse Javas, Contributions à la Faune des Indes Néderlandaises”, vol.1, 1-301, 1917. |
| [8] | G. H. Hardy, “Aphididae in Australia”, in Proceedings of the Royal Society of Queensland, pp.31-36, 1931. |
| [9] | V. F. Eastop, “A taxonomic study of Australian Aphidoidea (Homoptera)”, Australian Journal of Zoology, vol.14, pp.399-592, 1966. |
| [10] | V. F. Eastop, D. Hille Ris Lambers, “Survey of the World’s Aphids”. W. Junk, The Hague, 573 pp, 1976. |
| [11] | G. Remaudière, M. Remaudière, “Catalogue des Aphididae du monde/Catalogue of the world’s Aphididae”, Institut National de la Recherche Agronomique, Paris, pp. 473, 1997. |
| [12] | T. V. R. Ayyar, “Handbook of economic entomology for South India”, Government Press, Madras, pp. 516, 1963. |
| [13] | C. Siddappaji, D. R. N. Reddy, “A note on the occurrence of the aphid Pentalonia nigronervosa form caladii van der Goot (Aphididae-Homoptera) on cardamom (Elettaria cardamomum)”, Mysore Journal of Agricultural Science, vol. 6, pp.194-195, 1972. |
| [14] | M. Cermeli, “Lista actualizada de las especies de afidos (Homoptera: Aphidoidea) de Venezuela. Boletín de Entomología”, Venezolana N. S., vol.5, pp.183–187, 1990. |
| [15] | A. N. Basu, “One new genus and seven new species of aphids from Darjeeling District, West Bengal (Homoptera: Aphididae)”, Bulletin of Entomology, vol.9, pp.143–157, 1968. |
| [16] | D. Noordam, “Aphids of Java. Part VI”, Zoologische Verhandelingen, vol. 346, pp.85–212, 2004. |
| [17] | R. G. Foottit, H. E. E. Maw, K. S. Pike, R. H. Miller, “The identity of Pentalonia nigronervosa and P. caladii van der Goot (Hemiptera: Aphididae) based on molecular and morphometric analysis”, Zootaxa, vol.2358, pp.25-38, 2010. |
| [18] | P. Bhadra, B. K. Agarwala, “A comparison of fitness characters of two host plant-based congeneric species of the banana aphid, Pentalonia nigronervosa and P. caladi”, Journal of Insect Science, vol. 10, no. 152, insectscience.org/10.152, 2010. |
| [19] | H. D. Loxdale, P. Castanera, C. P. Brookes, "Electrophoretic study of enzymes from cereal aphid populations 1. Electrophoretic techniques and staining system for characterizing isozymes from six species of cereal aphids (Hemiptera: Aphididae)”, Bulletin of Entomological Research, vol.73, pp.645-657, 1983. |
| [20] | S. M. Singh, T. K. Cunningham, “Morphological and genetic differentiation in aphids (Aphididae)”, Canadian Entomologist, vol.113, pp.539-550, 1981. |
| [21] | J. D. Robson, M. G. Wright, R. P. P. Almeida, “Biology of Pentalonia nigronervosa (Hemiptera, Aphididae) on banana using different rearing methods”, Environmental Entomology, vol.36, pp.46–52, 2007. |
| [22] | D. J. Futuyama, T. E. Philippi, “Genetic variation and covariation in responses to host plant by Alsophila pometaria (Lepidoptera: Geometridae), Evolution, vol.41, pp.269-279, 1987. |
| [23] | S. Via, “Ecological genetics and host adaptations in herbivorous insects: the experimental study of evolution in natural and agricultural systems”, Annual Review of Entomology, vol.35, pp.421-446, 1990. |
| [24] | H. B. J. Lowe, “Variation in Myzus persicae (Sulzer) (Hemiptera: Aphididae) reared on different host plants”, Bulletin of Entomological Research, vol.62, pp.549-556, 1973. |
| [25] | S. Via, “The genetic structure of host plant adaptation in a spatial patchwork–demographic variability among reciprocally transplanted pea aphid clones”, Evolution, vol. 45, pp.827-852, 1991. |
| [26] | G. Görür, C. Lomonaco, A. Mackenzie, “Phenotypic plasticity in host-plant specialisation in Aphis fabae” Ecological Entomology, vol.30, pp.657-664, 2005. |
| [27] | G. Görür, “The importance of phenotypic plasticity in herbivorous insect speciation”. In: Insects and Phenotypic Plasticity, (ed. By T. N. Ananthakrishnan), Science publishers, Enfield, New Hampshire, pp.145-171, 2004. |
| [28] | R. K. Butlin, “Reinforcement: an idea evolving”, Trends in Ecology & Evolution, vol.10, pp.432-434, 2005. |
| [29] | G. Gorur, “The role of phenotypic plasticity in host race formation and sympatric speciation in phytophagous insects, particularly in aphids”, Turkish Journal of Zoology, vol.24, pp.63-68, 2005. |
| [30] | B. K. Agarwala, K. Das, “Host-plant based morphological, ecological & esterase variation in Aphis gossypii population (Homoptera: Aphididae)”, Entomon, vol.32, pp.89-95, 2007. |
| [31] | B. K. Agarwala,, K. Das, P. Ray Choudhury, “Morphological, ecological and biological variations in the mustard aphid, Lipaphis pseudobrassicae (Kaltenbach) (Hemiptera: Aphididae)”, Journal of Asia-Pacific Entomology, vol.12, pp.169-173, 2009. |
| [32] | B. K. Agarwala, “Phenotypic plasticity in aphids (Homoptera: Insecta): Components of variation and causative factors”, Current Science, vol.93, pp.308-313, 2007. |
| [33] | G. Görür, C. Lomonaco, A. Mackenzie, “Phenotypic plasticity in host choice behavior in black bean aphid, Aphis fabae (Momoptera: Aphididae)”, Arthropod Plant Interaction, vol.1, pp.187-194, 2007. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML