-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(3): 57-64
doi: 10.5923/j.als.20120203.03
Application of Fungicides Alternatives as Seed Treatment for Controlling Root Rot of Some Vegetables in Pot Experiments
N. S. El-Mougy, M. M. Abdel-Kader, M. D.E. Aly, S. M. Lashin
Plant Pathology Department, National Research Centre, Dokki 12622, Giza, Egypt
Correspondence to: N. S. El-Mougy, Plant Pathology Department, National Research Centre, Dokki 12622, Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Different root rot fungi, Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp. were isolated from various vegetables, i.e. Cucumber, Cantaloupe, Tomato and Pepper grown in plastic houses under protected cultivation system and showing root rot and or damping-off disease symptoms. The tested antagonistic fungi were Trichoderma harzianum, T. Viride and T. hamatum, Bacillus subtilis, Pseudomonas fluorescens and Saccharomyces cerevisiae. The efficacy of Calcium chloride, Thyme oil and /or bio-agents as seed dressing against disease incidence was evaluated in pot experiments under artificially infested with vegetables root rot causal organisms under greenhouse conditions. All applied treatments reduced significantly root rot incidence at both pre-, and post-emergence growth stages of Cucumber, Cantaloupe, Tomato and Pepper plants comparing with untreated check control. The obtained results showed that combination treatments of Calcium chloride, Thyme oil with bio-agents reduced significantly root rot incidence of all grown vegetables comparing with the application of each of them alone. Obtained results, in the present study, lead to suggest that integration between salt or essential oil and bio-agents is considered an applicable, safe and cost-effective method for controlling such soil-borne diseases.
Keywords: Root Rot, Cucumber, Cantaloupe, Tomato, Pepper, Essential Oil, Calcium Chloride, Thyme Oil, Bio-Agents, Biological Control
Article Outline
1. Introduction
- Root rot in vegetables strikes quickly and then ruin a whole crop. However the largest instruction course of actions is preventative measures, as therapy with fungicide does not normally work. Not all vegetables can arrangement root rot, but many standard vegetable crops are susceptible. Several fungi may cause root rot in vegetable plants, transmitting the disease through the soil. Some common fungi include Fusarium, Rhizoctonia, Sclerotinia, Pythium and others each of which causes a root rot named for the specific fungi that cause it. While the presence of one of these fungi is the primary cause for disease, plants exposed to poor growing conditions, such as soil that doesn't drain well, are most susceptible to root rot. The best way to avoid root rot is by eliminating these contributing causes, and practice sound cultivation techniques. The soil borne pathogens Rhizoctonia solani, Pythium ultimum, and Fusarium spp. can cause severe economic losses to field and greenhouse cucumber[1, 2]. Also, Fusarium stem and root rot on greenhouse long English Root rot disease occurs during the growing season of vegetable crops from seedling to flowering stages, and may cause pre-emergence infection. So far, apart from scientific and practical difficulties, there is no economic way to control the crop diseases.The management of soil-borne plant pathogens is particularly complex because these organisms live in or near the dynamic environment of the rhizosphere, and can frequently survive a long period in soil through the formation of resistant survival structures.The current management strategy relies on the intensive use of fungicides. In addition, chemical control does not give satisfactory control of the root disease. Application of biological control using antagonistic microorganisms has proved to be successful for controlling various plant diseases[5]. However, it is still not easy and costly in application. It can serve as the best control measure under greenhouse conditions. The concern of pesticides use with respect to human health and environment has brought increasing interest in alternatives use by avoiding negative effect on the environment. Essential oils are known for their natural components, such as mono terpenes, di terpenes, andhydro-carbons with various functional groups. Many other researchers have reported antifungal activities[6-8] of essential oils in food applications, pharmaceutical research and other areas as plant disease control.The present research focuses on finding compounds that are safe to humans and the environment, e.g. Calcium chloride and Thyme oil as well as biocontrol agents which may provide an alternative control of many soil and seed-borne pathogens. The objective of the present work was to evaluate fungicide alternatives and /or bio-agents against root rot incidence when used as seed treatment under greenhouse
2. Materials and Methods
2.1. Plant Materials
- Seeds of Cucumber (cv. Alpha), Cantaloupe (cv. Yatherb 7), Tomato (cv. Castel Rock) and Pepper (cv. California) were used in the present study.
2.2. Fungicides Alternatives
- The chemical salt, Calcium chloride (CaCl2) was obtained from El-Nasr Company for chemical industry, Egypt. Meanwhile, pure-grade of Thyme oil was obtained from Cairo Company for oils and aromatic extractions CID, Egypt. The essential oil was stored in dark glass bottles at 4℃.
2.3. Pathogenic Fungi
- The tested soilborne pathogenic fungi were Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp. These fungi were isolated from various vegetables, i.e. Cucumber, Cantaloupe, Tomato and Pepper grown in plastic houses under protected cultivation system and showing root rot and or damping-off disease symptoms[4]. The isolated fungi proved their aggressive ability to induce root rot disease of those vegetables.
2.4. Bio-agents
- The tested antagonistic fungi were Trichoderma harzianum, T. Viride and T. hamatum, B. subtilis, P. fluorescens and S. cerevisiae. These antagonists were isolated from cucumber, cantaloupe, tomato and pepper grown in plastic houses under protected cultivation systems and showing root rot disease symptoms[4]. The present bio-agents proved their antagonistic ability against the above mentioned pathogens.
2.5. Greenhouse Experiments
- Evaluation of Calcium chloride, Thyme oil and /or bio- agents seed dressing under artificial infestation with vegetables root rot causal organisms against disease incidence was performed in pot experiments under greenhouse conditions of Plant Pathology Dept., National Research Centre, Egypt.For root rot disease evaluation, experiments were carried out in a sandy loam soil artificially infested with root rot pathogens inocula. Inocula of pathogenic fungi, Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp. were individually grown on autoclaved sand barlly medium (1:1,v:v + 40% water) for two weeks at 25±1℃[9]. The fungal inocula were then mixed together to obtain a mixture contains equal share of tested pathogens. Soil infestation was carried out through amended with a mixture of root rot pathogens inocula (5% w:w) after[10], then mixed thoroughly to ensure equal distribution of pathogenic fungal inocula. Infested soil was then filled in plastic pots (30-cm- diameter) and irrigated every second day for 1 week before sowing.Evaluating the efficacy of Calcium chloride, Thyme oil, and/or bio-agents, i.e. T. harzianum, T. viride, B. subtilis, P. fluorescens and S. cerevisiae against root infection were applied as different treatments of seed dressing as follows: ● Calcium chloride 2% (20g/L).● Calcium chloride (2%) + T. harzianum (2x104cfu/mL).● Calcium chloride (2%) + T. viride (2x104cfu/mL).● Calcium chloride (2%) + B. subtilis (2x104cfu/mL).● Calcium chloride (2%) + P. fluorescens (2x104cfu/mL).● Calcium chloride (2%) + S. cerevisiae (2x104cfu/mL).● Thyme oil at the rate of 1% (10ml/L).● Thyme oil (1%) + T. harzianum (2x104cfu/mL).● Thyme oil (1%) + T. viride (2x104cfu/mL).● Thyme oil (1%) + B. subtilis (2x104cfu/mL).● Thyme oil (1%) + P. fluorescens (2x104cfu/mL).● Thyme oil (1%) + S. cerevisiae (2x104cfu/mL).● T. harzianum (2x104cfu/mL).● B. subtilis (2x104cfu/mL).● T. viride (2x104cfu/mL).● P. fluorescens (2x104cfu/mL).● S. cerevisiae (2x104cfu/mL).● Untreated controlSeeds of Cucumber, Cantaloupe, Tomato and Pepper were disinfested by emerging in sodium hypochlorite solution (3%) for 2 min, then rinsed and washed in sterilized water and air dried. Disinfected seeds were soaked for 3 h individually in the tested treatment solutions, and then left to air dry before sowing. Stock solutions (500 ml) of each of the tested treatment, Calcium chloride, Thyme oil and /or bio-agents were prepared in certain concentrations by dissolving in sterilized distilled water in form of sticky suspensions (1 mL of Arabic gum/100mL suspension). Two mL of Tween 80 (0.2% of water volume) were added to the essential oil solutions to obtain an aqueous emulsion. Tween 80 also known as polysorbate 80 is a nonionic surfactant and emulsifier derived from sorbitol obtained from various types of fruit. Polysorbate 80 is a water-soluble somewhat yellowish amber liquid that is used as a dispersing agent to mix oil and water and to solubilize fragrances and essential oils[11]. All stock solutions were prepared in black glass bottles kept in refrigerator until used. Coated seeds were sown as three seeds per pot, five pots per replicates in each treatment. Another set of soil amended only with a mixture of root rot pathogens (5% w:w) and sown with surface sterilized seeds was kept as control check treatment. The average percentage of root rot incidence at the pre- and post-emergence of growth stages was recorded up to 15 and 45 days of sowing date, respectively.Statistical analysisAll experiments were set up in a complete randomized design. One-way ANOVA was used to analyze differences between inhibitor effect and linear growth of pathogenic fungi in vitro. A general linear model option of the analysis system SAS[12] was used to perform the ANOVA. Duncan’s multiple range test at P < 0.05 level was used for means separation[13].
3. Results and Discussion
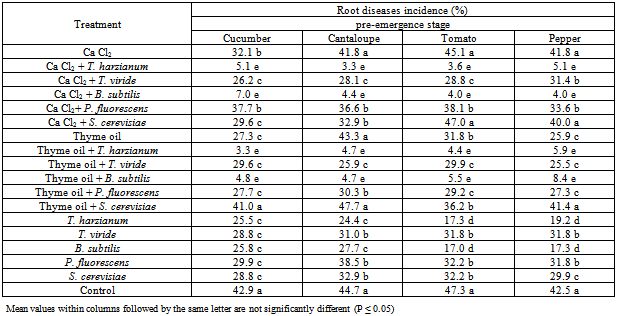
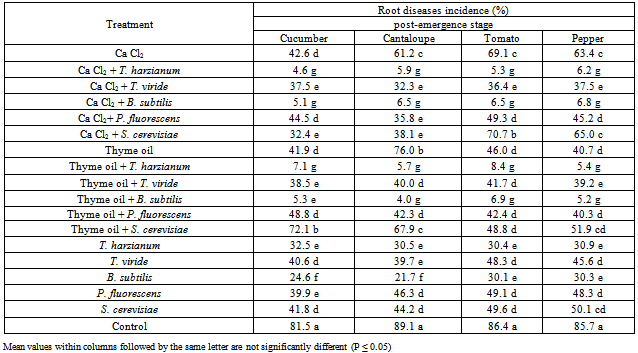
- The obtained results in Table (1) and Fig (1) showed the efficacy of applied Calcium chloride, Thyme oil and /or bio-agents as seed treatment against tested vegetables root rot diseases incidence. Presented data revealed that all applied treatments reduced significantly root rot incidence at both pre-, and post-emergence growth stages of Cucumber, Cantaloupe, Tomato and Pepper plants comparing with untreated check control. Data also showed that combination treatments of Calcium chloride, Thyme oil with bio-agents reduced significantly root rot incidence of all grown vegetables comparing with the application of each of them alone. At pre-emergence growth stage the treatments of the bio-agents, T. harzianum and B. subtilis recorded higher significant reduction in root rot incidence when combined with calcium chloride (3.3-7.0%), Thyme oil (3.3-8.4%) and/or applied alone (17.3-25.5% & 17.0-25.8%) comparing with the other bio-agents P. fluorescens (33.6-38.1% & 27.3-30.3% & 31.8-38.5%) and S. cerevisiae (29.6-47.0% & 36.2-47.7% & 28.8-32.9%), in respective order. Meanwhile, the root rot incidence for Cucumber, Cantaloupe, Tomato and Pepper were 42.9, 44.7, 47.3 and 42.5%, respectively in untreated seeds check treatment. Data in Table (1) and Fig. (1) revealed that treatments of T. harzianum and B. subtilis combined with either calcium chloride or Thyme oil seem to have the superior treatments for controlling root rot disease incidence of tested vegetables.Also, data in Table (1) showed another feature concerning S. cerevisiae and P. fluorescens that application of S. cerevisiae treatment alone was significantly reduced root rot incidence comparing with combination of either Calcium chloride or Thyme oil treatments, while when P. fluorescens combined with Thyme oil resulted in significant reduction in root rot incidence comparing with either its application alone or when combined with Calcium chloride, respectively. Regarding T. viride treatments, recorded results in Table (1) showed fluctuated response against root rot incidence for individual host plant. No significant differences were observed between T. viride treatments in cucumber, while T. viride combined with calcium chloride and Thyme oil were significantly differed than T. viride alone for reducing disease incidence of Cantaloupe and Tomato.As for Pepper, T. viride combined with Thyme oil reduced significantly root rot incidence followed by T. viride combined with calcium chloride or applied alone. At post- emergence of tested vegetables growth stage (Table, 2 & Fig. 2), treatments of the bio-agents, T. harzianum and B. subtilis combined with calcium chloride, Thyme oil and/or applied alone were significantly caused higher protection against root rot disease incidence.Also, treatment of S. cerevisiae alone was significantly reduced disease incidence of Cucumber, Cantaloupe and Pepper followed by its combination with Calcium chloride and Thyme oil, while no significant differences were observed between these treatments with Tomato plants, although they differed significantly with untreated check control treatment. The other bio-agents, T. viride and P. fluorescens combined with either Calcium chloride or Thyme oil had superior effect for reducing root rot incidence comparing with their application alone. In general data in Table (1 and 2) and fig (1 and 2) revealed that application of T. harzianum and B. subtilis either alone or combined with Calcium chloride and Thyme oil proved to be the most significant seed treatments for controlling root rot incidence of Cucumber, Cantaloupe, Tomato and Pepper in pot experiment. Similar results were also recorded by many investigators. Within the large reservoir of natural fungicides that exist in plants examples exist that would serve as safe and effective alternatives to synthetic fungicides. Such compounds (volatile components, essential oils), if properly formulated and applied, could be used directly or could serve as templates for synthetic analogs.In the present study application of Thyme oil as seed coating revealed its efficacy against seed or plant invasion under in vivo conditions which resulted in a significant reduction in root rot incidence of grown vegetables under greenhouse conditions. The suppression of root rot development under greenhouse condition seems to corresponds with the ability of this essential oil to reduce disease incidence. The major components of the used Thyme oil are carvacrol and thymol. These results could be correlated with the stability and concentration as well as volatility of the active component in the essential oil used. Essential oils from oregano and thyme for 24 h as fumigants were applied against the mycelia and spores of Aspergillus flavus, Aspergillus niger and Aspergillus ochraceus, as well as against natural microflora of wheat grains[14]. Also, they recorded that their findings emphasize the toxicity of oregano and thyme essential oils as fumigants against fungi attacking stored grain and strengthen the possibility of using them as an alternative to chemicals for preserving stored grains.Moreover, it was recorded that that in poison food medium thyme oil had greatest antifungal activity against Penicillium digitatum and Rhizopus stolonifer at 1,000 and ≥600 µL/L, respectively[15]. In vapour phase, thyme oil at ≥5 µL completely inhibited the mycelial growth of pathogens.Thyme essential oil was tested against the fungi, Aspergillus, Penicillium, Alternaria, Ulocladium, Absidia, Mucor, Cladosporium, Trichoderma,Rhizopus and Chaetomium [16]. They found that the vaporous phase of the thyme essential oil (82 μg l−1) in glass chambers strongly suppressed the sporulation of moulds during 60 days of exposure. Moreover, [17] reported that Thyme and Egyptian geranium oil inhibited growth of all test fungi, Aspergillus niger, Trichoderma viride, and Penicillium chrysogenum on southern yellow pine for 20 weeks. Similar results were also reported by many researchers indicating the efficacy of essential oils as antifungal inhibitors for a large number of soilborne pathogens[18-21].The mode by which microorganisms are inhibited by essential oils and their chemical compounds seem to involve different mechanisms. It has been hypothesized that the inhibition involves phenolic compounds, because these compounds sensitize the phospholipid bilayer of the microbial cytoplasmic membrane causing increased permeability and unavailability of vital intracellular constituents[22].
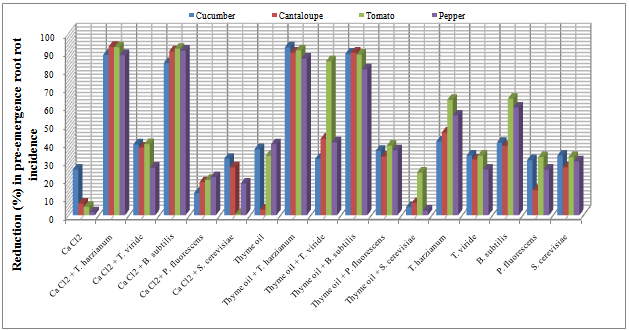 | Figure 1. Reduction in vegetables root diseases (Pre -emergence) caused by soil-borne pathogenic fungi in response to applying Calcium chloride, Thyme oil and /or bio-agents as seed treatment under open greenhouse conditions |
|
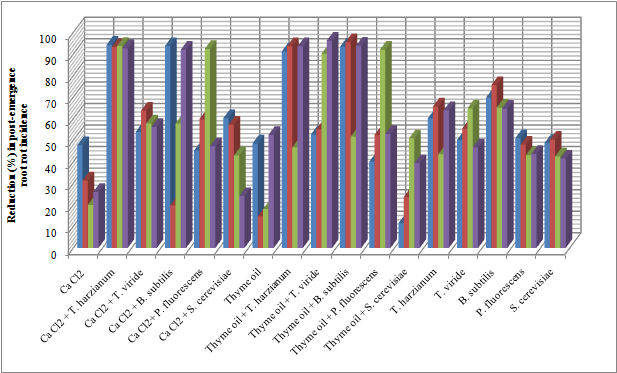 | Figure 2. Reduction in vegetables root diseases (Post-emergence) caused by soil-borne pathogenic fungi in response to applying Calcium chloride, Thyme oil and /or bio-agents as seed treatment under open greenhouse conditions |
ACKNOWLEDGMENTS
- This work was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No. 1059.
References
| [1] | Kaulizakis, M., 1997. National Agricultural Foundation, Sub-tropical plant and olive trees. Instt. China Lab. Pl. Pathol. Agrokipio Chinia Crete Greece, 4, 383–6 |
| [2] | Roberts, D.P., Lohrke, S.M., Meyer, S.L.F., Buyer, J.S., Bowers, J.H., 2005, Bio-control agents applied individually and in combination for suppression of soil borne diseases of cucumiber. Crop Prot., 24, 141–155 |
| [3] | Erol, F.Y., Tunali, B., 2009, Determination of root and crown rot diseases in tomato growing area of Samsun province. Acta Hort. (ISHS), 808, 65-70. |
| [4] | El-Mougy, N.S., Abdel-Kader, M.M., Abdel-Kareem, F., Embaby, E.I., El-Mohamady, R., Abd El-Khair, H., 2011, Survey of Fungal Diseases Affecting Some Vegetable Crops and Their Rhizospheric Soilborne Microorganisms Grown under Protected Cultivation System in Egypt. Research Journal of Agriculture and Biological Sciences, 7(2), 203-211. |
| [5] | Sivan, A., 1987, Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Disease 71, 587--592. doi: 10.1094/PD-71-0587 |
| [6] | Muller-Riebau, F., Berger, B., Yegen, O., 1995, Chemical composition and fungitoxic properties to phytopathogenic fungi of essential oil of selected aromatic plants growing wild in Turkey. Journal of Agricultural and Food Chemistry, 43, 2262–2266. |
| [7] | Deferera, D.J., Ziogas, B.N., Polissiou, M.G., 2000, GC-MS Analysis of essential oil from some Greek aromatic plants and their fungitoxicity on Pennicillium digitatum. Journal of Agricultural and Food Chemistry, 48, 2576–2581. |
| [8] | Sridhar, S.R., Rajagopal, R.V., Rajavel, R., Masiilamani, S., Narasimhan, S., 2003, Antifungal activity of some essential oils. Journal of Agricultural and Food Chemistry, 512, 7596–7599. |
| [9] | Abdel-Kader, M.M., 1997, Field application of Trichoderma harzianum as biocide for control bean root rot disease. Egypt. J. Phytopathol., 25, 19–25. |
| [10] | Abdel-Kader, M.M., 1999, Biological and chemical control of wilt disease of hot pepper (Capsicum annum L.). Egypt. J. Phytopathol., 27,1-8. |
| [11] | Anonymous, 2007, Tween 80-Polysorbate 80. Well Naturally products LTD. http://www.wellnaturally.ca/ingredients/ tween80.html |
| [12] | SAS Institute Inc., 1996, SAS/STAT User’s Guide. Version 6, 12th ed. Vol. 2, SAS Institute, Inc. Cary, North Carolina, USA, 846 pp. |
| [13] | Winer B.J., 1971, Statistical Principles in Experimental Design. 2nd ed. MiGraw- Hil Kogakusha, LTD, 596 pp. |
| [14] | Paster, N., Menasherov, M. Ravid, U., Juven, B., 1995, Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J food prot., 58 (1), 81-85. |
| [15] | Abdollahi, A., Hassani, A., Ghosta, Y., Meshkatalsadat, M.H., Shabani, R., 2011, Screening of antfungal properties of essential oils extracted from sweet basil, fennel summer savory and thyme against post harvest phytopathogenic fungi. Journal of Food Safety, 31, 350–356. doi: 10.1111/j.1745-4565.2011.00306.x |
| [16] | Segvic Klaric, M., Kosalec, I., Mastelic, J., Pieckova, E., Pepeljnak, S., 2007, Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Letters in Applied Microbiology, 44, 36–42. doi: 10.1111/j.1472-765X.2006.02032.x |
| [17] | Yang, V.W., Clausen, C.A., 2007, Antifungal effect of essential oils on southern yellow pine. International Biodeterioration & Biodegradation, 59, 302–306. doi:10.1016/ j.ibiod.2006.09.004 |
| [18] | Akgul A., Kivanç, M., 1988, Inhibitory effect of selected Turkish spices and oregano components on some foodborne fungi. Intern. J. Food Microbiol., 6, 263–268. |
| [19] | Kumar A., Tripathi, S.C., 1991, Evaluation of the leaf juice of some higher plants for their toxicity against soilborne pathogens. Plant and Soil, 132, 297–301. |
| [20] | Singh U.P., Chauhan, V.B., Wagner, K.G., Kumas, A., 1992, Effect of ajoene, a compound derived from garlic (Allium sativum), on Phytophthora drechsleri f. sp. cajani. Mycologia, 84, 105–108. |
| [21] | Singh U.P., Singh, K.P., Tripathi, V.K. Pandy, V.B., 1994, Antifungal activity of some naturally occurring plant alkaloids. Intern. J. Trop. Plant Dis., 12, 209–212. |
| [22] | Juven B.J., Kanner, J., Sched, F., Weisslowicz, H., 1994, Factors that interact with the antibacterial of thyme essential oil and its active constituents. J. Appl. Microbiol., 76, 626–631. |
| [23] | Lanciotti R., Gianotti A., Patrignani N., Belleti N., Guerzoni M.E., Gardini F., 2004, Use of natural aroma compounds to improve shelf-life of minimally processed fruits. Trends Food Sci. Technol. 15, 201–208. |
| [24] | Abdel-Kader, M.M., El-Mougy, N.S., Lashin, S.M., 2011, Essential oils and Trichoderma harzianum as an integrated control against Faba Bean root rot pathogens. Journal of Plant Protection Research, 51(3), 306-313. |
| [25] | Abd-Alla, M.A., El-Mougy, N.S., El-Gamal, N.G., 2009, Formulation of essential oils and yeast for controlling postharvest decay of tomato fruits. Plant Pathol. Bull., 18, 23-33. |
| [26] | Chao W.L., Nelson, E.B., Harman, G.E., Hoch, H.C., 1986, Colonization of the rhizosphere by biological control agents applied to seeds. Phytopathology, 76, 60–65. |
| [27] | El-Mougy, N.S., 2001, Field application of certain biological and chemical approaches on controlling Bean wilt disease. Egypt. J. Phytopathol., 29, 69–78. |
| [28] | Wright B., Rowse, H.R., Whipps, J.M., 2003, Application of beneficial microorganisms to seeds during drum priming. Biocontrol Sci. Technol., 13, 599–614. Doi: 10.1080/ 09583150310001517992 |
| [29] | El-Mougy N.S., Abdel-Kader, M.M., 2008, Long term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Aust. Plant Pathol., 37 (5), 464–471. |
| [30] | Yigit, F., Dikilitas, M., 2007, Control of Fusarium Wilt of Tomato by Combination of Fluorescent Pseudomonas, Non-pathogen Fusarium and Trichoderma harzianum T-22 in Greenhouse Conditions. Plant Pathology Journal, 6 (2), 159-163. |
| [31] | Droby, S., Wisniewski, M.E., Cohen, L., Weiss, B., Touitou, D., Eilam, Y., Chalutz, E., 1997, Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii. Plant Dis., 87, 310–315. |
| [32] | McLaughlin R.J., Wisniewski, M.E., Wilson, C.L., Chalutz, E., 1990, Effect of inoculum concentration and salt solutions on biological control of postharvest diseases of apple with Candida sp. Phytopathology, 80, 456–461. |
| [33] | Conway S.W., Sams C.E., McGuire R.G., Kelman A., 1992, Calcium treatment of apples and potatoes to reduce postharvest decay. Plant Dis. 76, 329–333. |
| [34] | Conway S.W., Sams C.E., Wang C.Y., Abbott J.A., 1994, Additive effects of postharvest calcium and heat treatments on reducing decay and maintaining quality in apples. J. Am. Soc. Hortic. Sci., 119, 49–53. |
| [35] | Poovaiah B.W., 1986, Role of calcium in prolonging storage life of fruits and vegetables. Food Technol., 40, 86–89. |
| [36] | Saftner R.A., Conway, W.S., Sams, C.E., 1997, Effects of some polyamine biosynthesis inhibitors and calcium chloride on in vitro growth and decay development in apples caused by Botrytis cinerea and Penicillium expansum. J. Am. Soci. Hort. Sci., 122, 380–385. |
| [37] | El-Mougy, N.S., Abdel-Kader, M.M., 2009, Salts application for suppressing potato early blight. Journal of Plant Protection Research, 49 (4), 353-361. |
| [38] | Conway, W.S., Sams, C.E., 1984, Possible mechanisms by which postharvest calcium treatment reduces decay in apples. Phytopathology, 74, 208-210. |
| [39] | Biggs, A.P., Peterson, C.A., 1990, Effect of chemical applications to peach bark wounds on acccmulation of lignin and subrin and susceptibility to Leucostoma persoonii. Phytopathology, 80, 861-865. |
| [40] | Biggs, A.R., Inglee, M., Solihati, W,D., 1993, Control of Alternaria infection of fruit of apple cultivar Nittany with calcium chloride and fungicides. Plant Dis., 77, 399-403. |
| [41] | Bateman, D.F., Lumsden, R.D., 1965, Relation of calcium content and nature of the pectic substances in bean hypocotyles of different ages to susceptibility of an isolate of Rhizoctonia solani. Phytopathology, 55, 734-738. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML